Which of the following molecules has an assumed van't Hoff factor equal to 2? A. KPO4 B. M9SO4 C. NAHSO4 D. CH1206 When CH:OH is dissolved in water, how many particles are in the solution? A. 1 B. 3 C. 5
Which of the following molecules has an assumed van't Hoff factor equal to 2? A. KPO4 B. M9SO4 C. NAHSO4 D. CH1206 When CH:OH is dissolved in water, how many particles are in the solution? A. 1 B. 3 C. 5
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.98QE
Related questions
Question
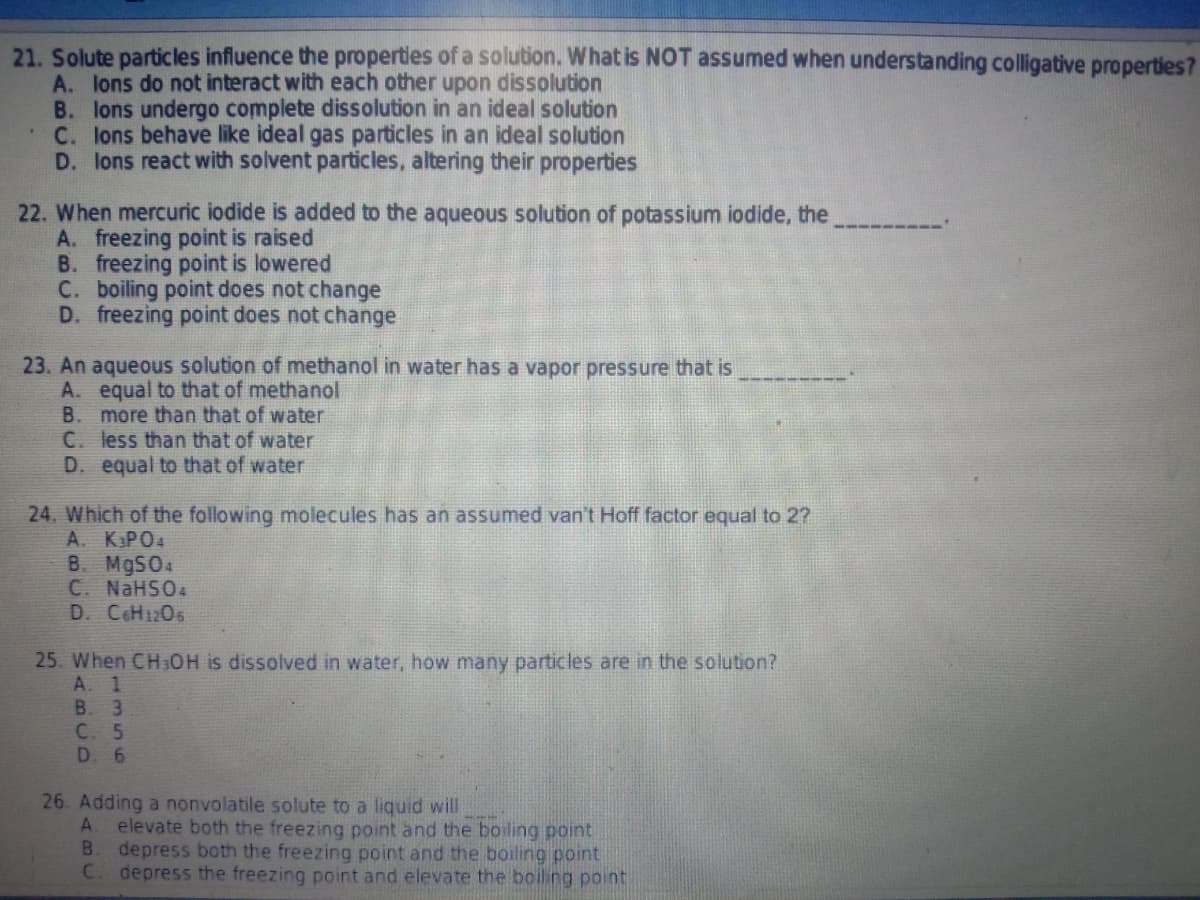
Transcribed Image Text:21. Solute particles influence the properties of a solution. What is NOT assumed when understanding colligative properties?
A. lons do not interact with each other upon dissolution
B. lons undergo complete dissolution in an ideal solution
C. lons behave like ideal gas particles in an ideal solution
D. lons react with solvent particles, altering their properties
22. When mercuric iodide is added to the aqueous solution of potassium iodide, the
A. freezing point is raised
B. freezing point is lowered
C. boiling point does not change
D. freezing point does not change
23. An aqueous solution of methanol in water has a vapor pressure that is
A. equal to that of methanol
B.
more than that of water
C. less than that of water
D. equal to that of water
24. Which of the following molecules has an assumed van't Hoff factor equal to 2?
A. KPO4
B. M9SO4
C. NaHSO4
D. CoH1206
25. When CH3OH is dissolved in water, how many particles are in the solution?
A. 1
B. 3
C. 5
D. 6
26. Adding a nonvolatile solute to a liquid will
A.
elevate both the freezing point and the boiling point
B.
depress both the freezing point and the boiling point
C. depress the freezing point and elevate the boiling point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning