Which of the following pairs of compounds will react to form a precipitate? Why? calcium chloride and silver nitrate sodium iodide and potassium chloride calcium chloride and sodium nitrate Your explanation should be written in complete sentences.
Which of the following pairs of compounds will react to form a precipitate? Why? calcium chloride and silver nitrate sodium iodide and potassium chloride calcium chloride and sodium nitrate Your explanation should be written in complete sentences.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter8: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 6A
Related questions
Question
100%
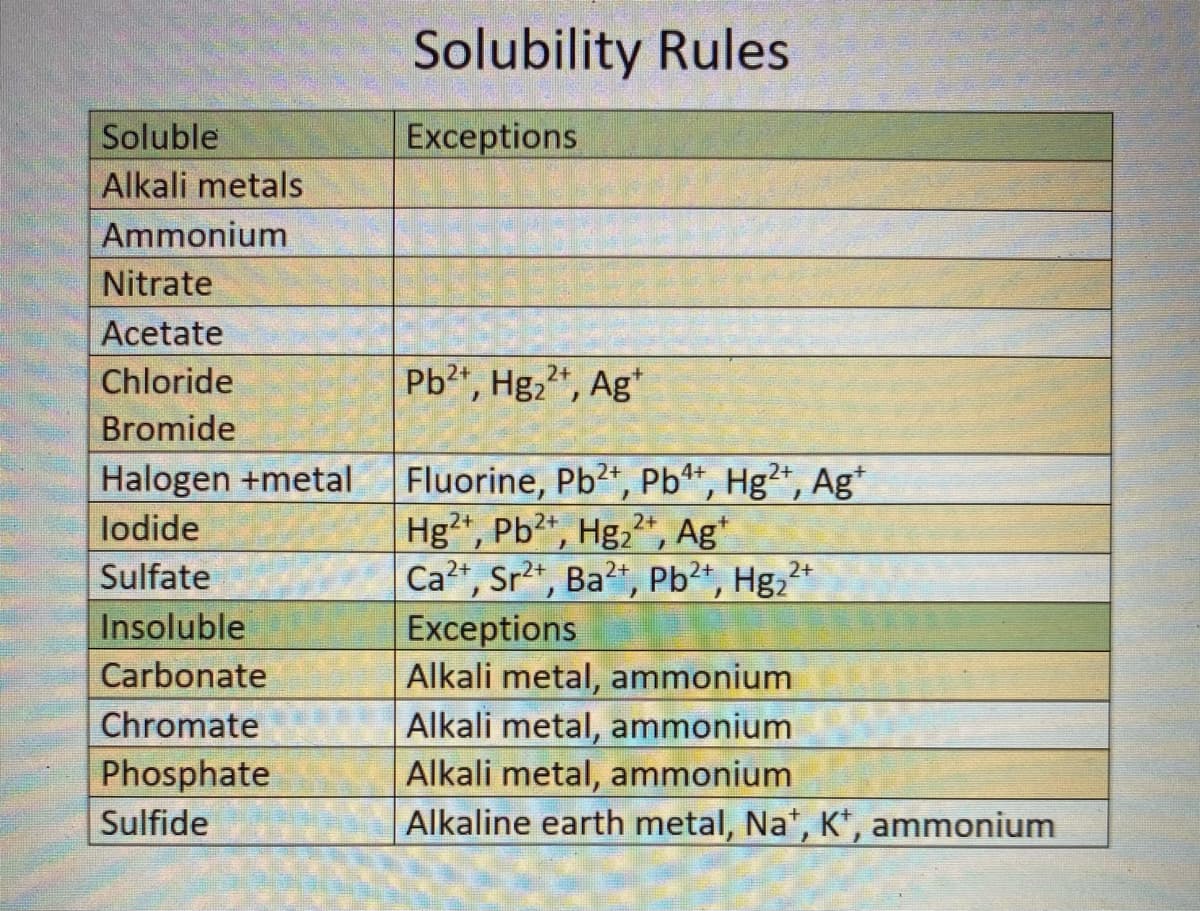
Transcribed Image Text:Solubility Rules
Soluble
Exceptions
Alkali metals
Ammonium
Nitrate
Acetate
Chloride
Pb2, Hg2", Ag*
Bromide
Fluorine, Pb2, Pb**, Hg²*, Ag*
Hg²*, Pb?*, Hg,*, Ag*
Ca?t, Sr2t, Ba?", Pb²*, Hg,2+
Halogen +metal
4+
lodide
2+
2+
2+
Sulfate
Insoluble
Exceptions
Alkali metal, ammonium
Alkali metal, ammonium
Alkali metal, ammonium
Alkaline earth metal, Na*, K, ammonium
Carbonate
Chromate
Phosphate
Sulfide

Transcribed Image Text:Question 27
Which of the following pairs of compounds will react to form a precipitate? Why?
calcium chloride and silver nitrate
sodium iodide and potassium chloride
calcium chloride and sodium nitrate
Your explanation should be written in complete sentences.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER