Which of the following statements about buffers is (are) true? O (a) the pH of a buffered solution remains unchanged no matter how much acid or base is added to the solution. (b) the strongest buffers are made up of strong acids and strong bases (c) a buffer composed of a weak acid of pKa = 5 is a better buffer at pH 4 than at pH 6.00 (d) when pH = pka, the weak acid and conjugate base concentrations in a buffer are equal %3D (e) both (b) and (d)
Which of the following statements about buffers is (are) true? O (a) the pH of a buffered solution remains unchanged no matter how much acid or base is added to the solution. (b) the strongest buffers are made up of strong acids and strong bases (c) a buffer composed of a weak acid of pKa = 5 is a better buffer at pH 4 than at pH 6.00 (d) when pH = pka, the weak acid and conjugate base concentrations in a buffer are equal %3D (e) both (b) and (d)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 4RQ: A good buffer generally contains relatively equal concentrations of weak acid and conjugate base. If...
Related questions
Question
Question 6
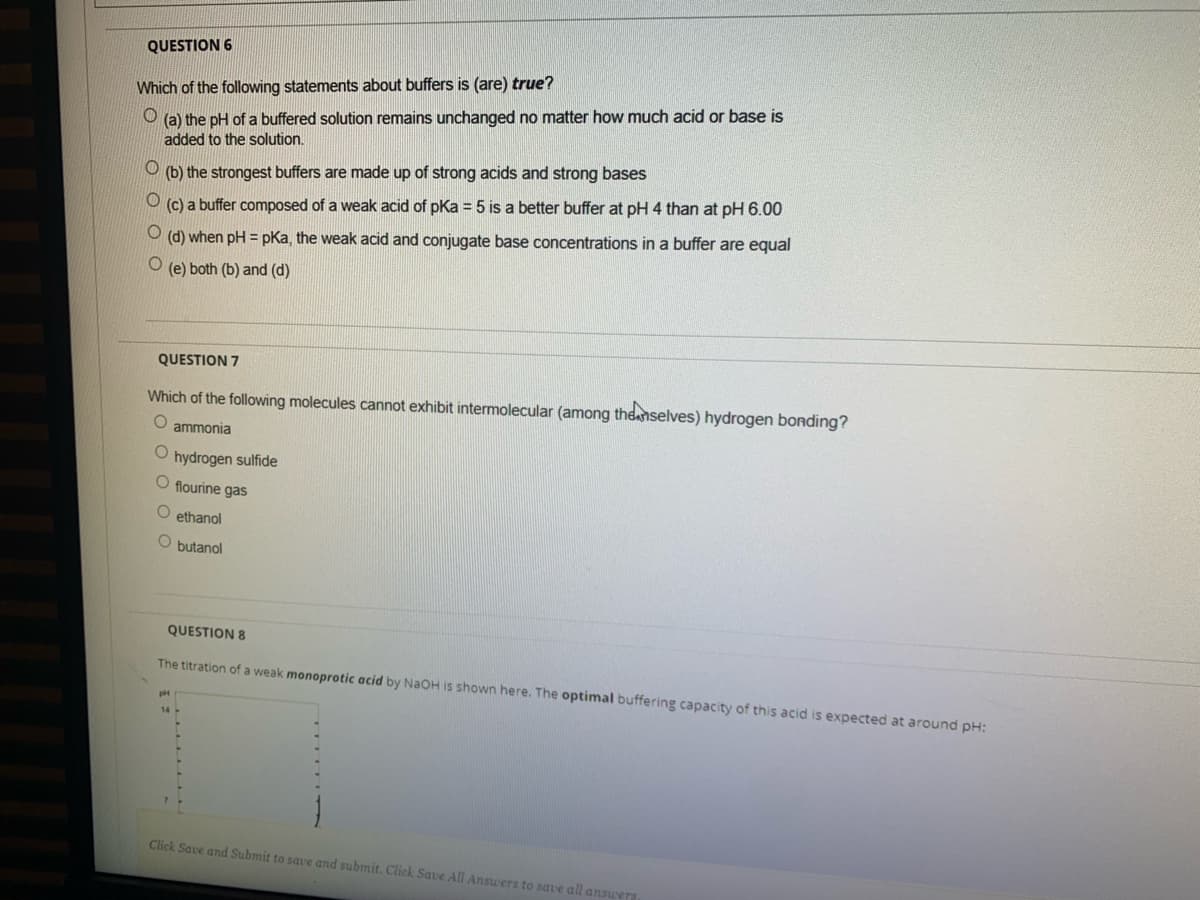
Transcribed Image Text:QUESTION 6
Which of the following statements about buffers is (are) true?
O (a) the pH of a buffered solution remains unchanged no matter how much acid or base is
added to the solution.
(b) the strongest buffers are made up of strong acids and strong bases
(c) a buffer composed of a weak acid of pKa = 5 is a better buffer at pH 4 than at pH 6.00
(d) when pH = pKa, the weak acid and conjugate base concentrations in a buffer are equal
(e) both (b) and (d)
QUESTION 7
Which of the following molecules cannot exhibit intermolecular (among the nselves) hydrogen bonding?
ammonia
O hydrogen sulfide
O flourine gas
O ethanol
O butanol
QUESTION 8
The titration of a weak monoprotic acid by NAOH is shown here. The optimal buffering capacity of this acid is expected at around pH:
Click Save and Submit to save and submit. Click Save All Answers to save all answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning