Which of the following statements are true? Select all that apply. O For molecules with varying molecular weights, the dispersion forces may not become stronger as the molecules become more polarizable. O The smaller the atom, the less polarizable it is. O In terms of the total attractive forces for a given substance, the contribution of dipole-dipole interactions, when present, are always larger than the contributions of dispersion forces. O For the halogens, the dispersion forces increase while the boiling points decrease as you go down the column in the periodic table from F2 to I2 O All other factors being the same, dispersion forces between linear molecules are greater than dispersion forces between molecules whose shapes are nearly spherical.
Which of the following statements are true? Select all that apply. O For molecules with varying molecular weights, the dispersion forces may not become stronger as the molecules become more polarizable. O The smaller the atom, the less polarizable it is. O In terms of the total attractive forces for a given substance, the contribution of dipole-dipole interactions, when present, are always larger than the contributions of dispersion forces. O For the halogens, the dispersion forces increase while the boiling points decrease as you go down the column in the periodic table from F2 to I2 O All other factors being the same, dispersion forces between linear molecules are greater than dispersion forces between molecules whose shapes are nearly spherical.
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 1RQ: What are intermolecular forces? How do they differ from intramolecular forces? What are...
Related questions
Question
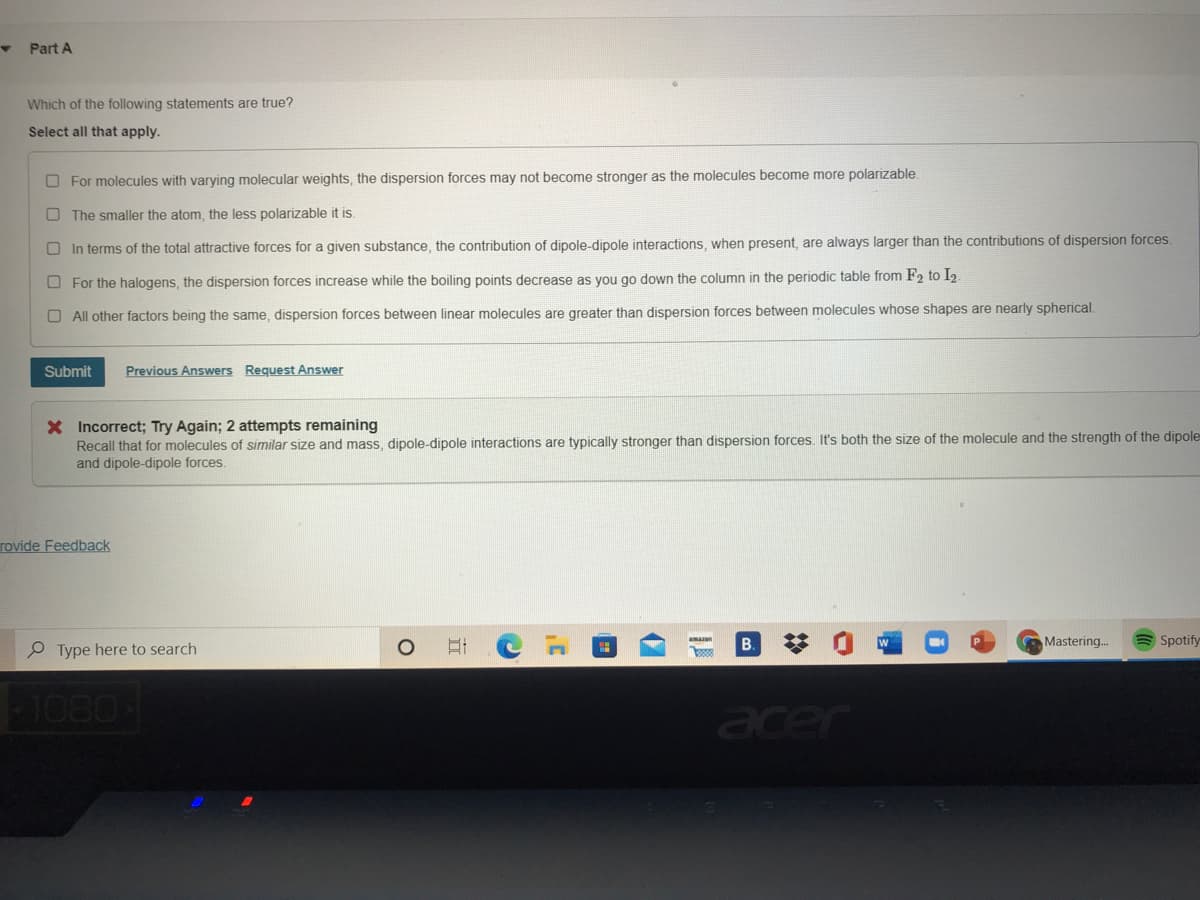
Transcribed Image Text:Part A
Which of the following statements are true?
Select all that apply.
O For molecules with varying molecular weights, the dispersion forces may not become stronger as the molecules become more polarizable
O The smaller the atom, the less polarizable it is.
O In terms of the total attractive forces for a given substance, the contribution of dipole-dipole interactions, when present, are always larger than the contributions of dispersion forces.
O For the halogens, the dispersion forces increase while the boiling points decrease as you go down the column in the periodic table from F2 to I2.
O All other factors being the same, dispersion forces between linear molecules are greater than dispersion forces between molecules whose shapes are nearly spherical.
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 2 attempts remaining
Recall that for molecules of similar size and mass, dipole-dipole interactions are typically stronger than dispersion forces. It's both the size of the molecule and the strength of the dipole
and dipole-dipole forces.
rovide Feedback
B.
Mastering.
Spotify
P Type here to search
1080
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning