Which of the following would be expected to form hydrogen bonds with water? (Select all that apply.) propanoic acid, t +4 +41 ethyl methyl ketone, N-methylacetamide, Ⓒhexane, None of the Above -H -H 4
Which of the following would be expected to form hydrogen bonds with water? (Select all that apply.) propanoic acid, t +4 +41 ethyl methyl ketone, N-methylacetamide, Ⓒhexane, None of the Above -H -H 4
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 60P
Related questions
Question
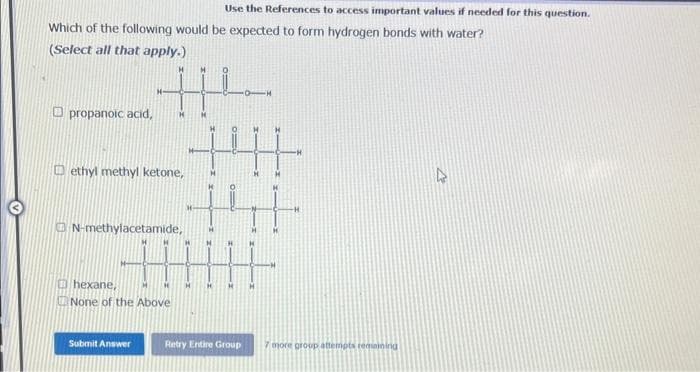
Transcribed Image Text:Use the References to access important values if needed for this question.
Which of the following would be expected to form hydrogen bonds with water?
(Select all that apply.)
propanoic acid, H
ethyl methyl ketone,
N-methylacetamide,
hexane,
None of the Above
Submit Answer
14
H
Retry Entire Group
7 more group attempts remaining
27
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning