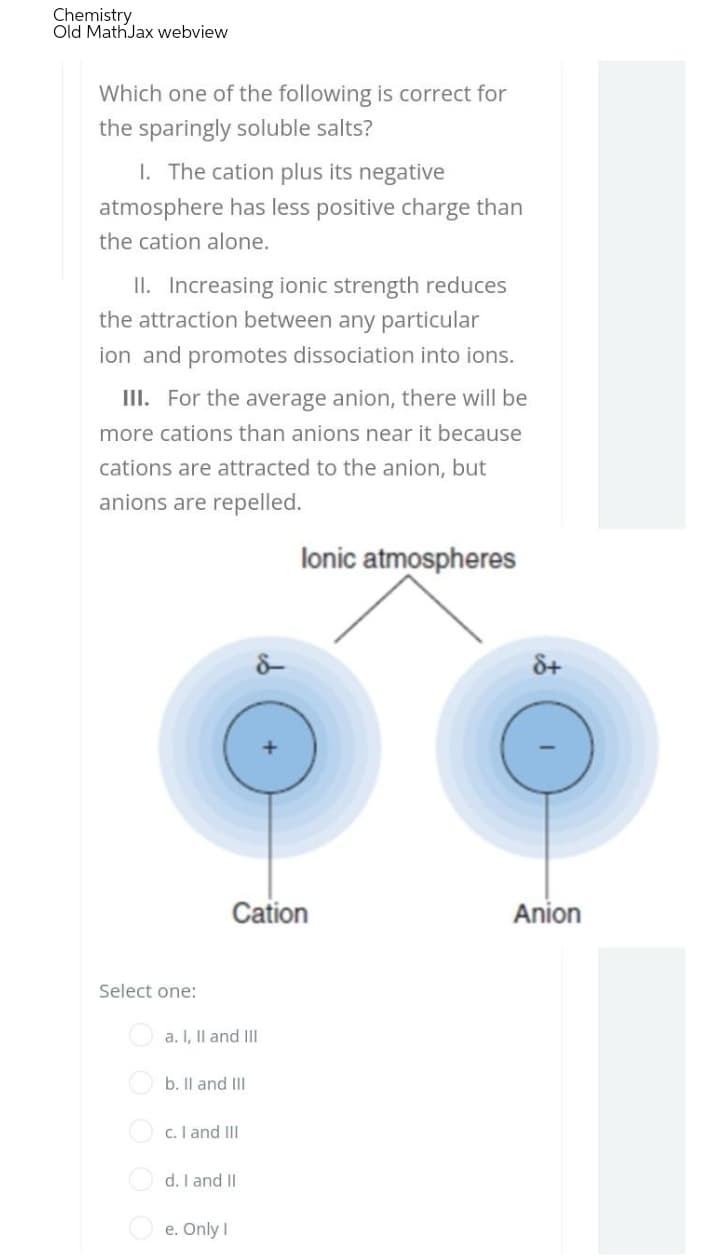
Which one of the following is correct for the sparingly soluble salts? 1. The cation plus its negative atmosphere has less positive charge than the cation alone. II. Increasing ionic strength reduces the attraction between any particular ion and promotes dissociation into ions. III. For the average anion, there will be more cations than anions near it because cations are attracted to the anion, but anions are repelled.
Which one of the following is correct for the sparingly soluble salts? 1. The cation plus its negative atmosphere has less positive charge than the cation alone. II. Increasing ionic strength reduces the attraction between any particular ion and promotes dissociation into ions. III. For the average anion, there will be more cations than anions near it because cations are attracted to the anion, but anions are repelled.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 4P: Figure 16.3 shows the solubility of AgNO3 in water inunits of moles of AgNO3 per kilogram of H2O. If...
Related questions
Question
Transcribed Image Text:Chemistry
Old MathJax webview
Which one of the following is correct for
the sparingly soluble salts?
1. The cation plus its negative
atmosphere has less positive charge than
the cation alone.
II. Increasing ionic strength reduces
the attraction between any particular
ion and promotes dissociation into ions.
III. For the average anion, there will be
more cations than anions near it because
cations are attracted to the anion, but
anions are repelled.
lonic atmospheres
Select one:
Cation
a. I, II and III
b. II and III
c. I and III
d. I and II
e. Only I
d+
Anion
Transcribed Image Text:With explainations please am not in hurry (6)
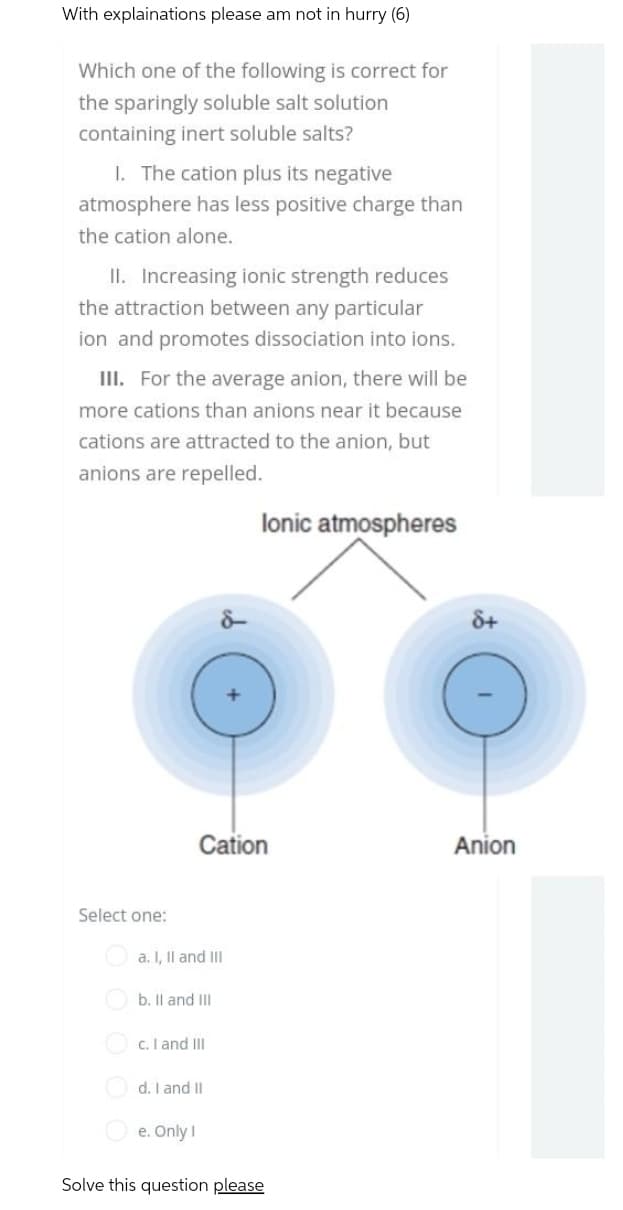
Which one of the following is correct for
the sparingly soluble salt solution
containing inert soluble salts?
1. The cation plus its negative
atmosphere has less positive charge than
the cation alone.
II. Increasing ionic strength reduces
the attraction between any particular
ion and promotes dissociation into ions.
III. For the average anion, there will be
more cations than anions near it because
cations are attracted to the anion, but
anions are repelled.
lonic atmospheres
Select one:
Cation
a. I, II and III
b. II and III
c. I and III
d. I and II
e. Only I
Solve this question please
8+
Anion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning