Which one of the following molecules would have the largest bond dissociation energy if all bonds in the molecule are broken? Single H C|N Bond н 432 А) СО 411 346 N 386 305 167 B) N2 459 358 201 142 C=C 602 C=O 799 C) O2 C=C 835 C=0 1072 Multiple Bonds 615 D) HCN C=N O=0 494 C=N 887 N=N 942 **All values in kJ/mol**
Which one of the following molecules would have the largest bond dissociation energy if all bonds in the molecule are broken? Single H C|N Bond н 432 А) СО 411 346 N 386 305 167 B) N2 459 358 201 142 C=C 602 C=O 799 C) O2 C=C 835 C=0 1072 Multiple Bonds 615 D) HCN C=N O=0 494 C=N 887 N=N 942 **All values in kJ/mol**
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 65SCQ: Three of the four molecular orbitals for cyclobutadiene are pictured here. Place them in order of...
Related questions
Concept explainers
Bond Parameters
Many factors decide the covalent bonding between atoms. Some of the bond parameters are bond angle, bond order, enthalpy, bond length, etc. These parameters decide what kind of bond will form in atoms. Hence it is crucial to understand these parameters in detail and understand how changing these parameters affects the kind of bonding or various characteristics.
Bond Dissociation Energy
The tendency of an atom to attract an electron is known as its electronegativity.
Question
Which one of the following molecules would have the largest bond dissociation energy if all bonds in the molecule are broken?
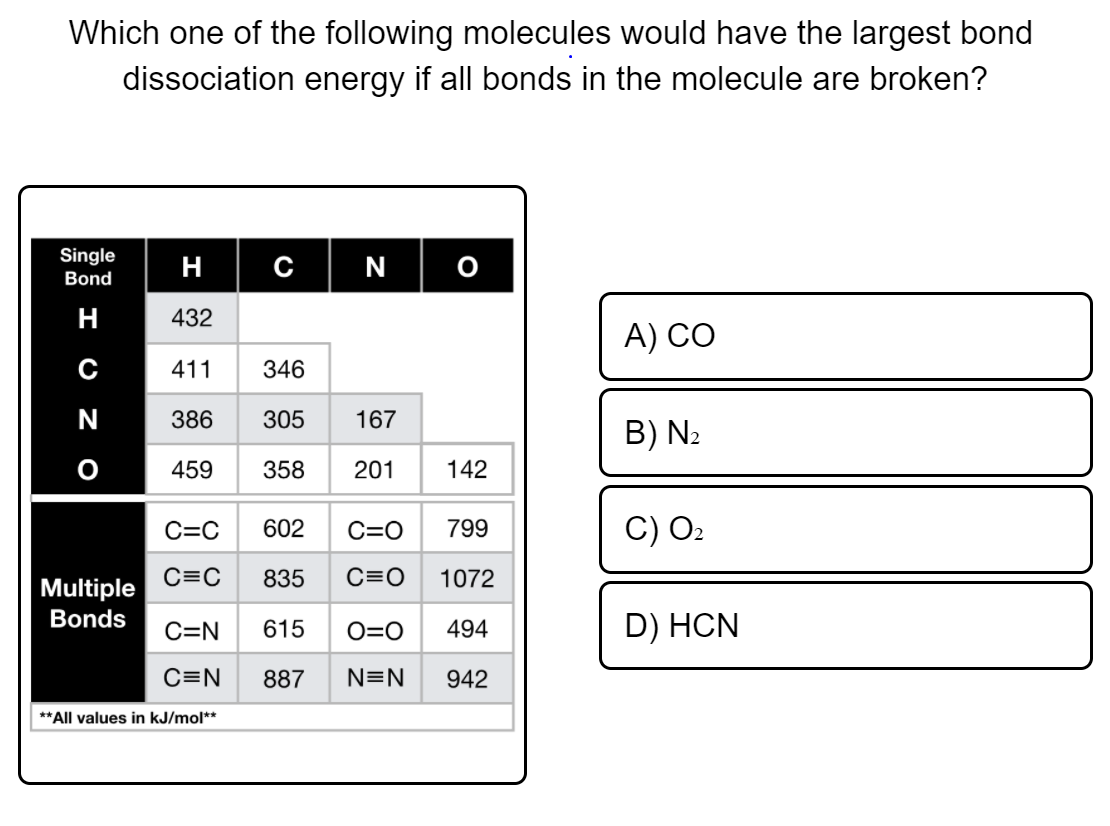
Transcribed Image Text:Which one of the following molecules would have the largest bond
dissociation energy if all bonds in the molecule are broken?
Single
H C|N
Bond
н
432
А) СО
411
346
N
386
305
167
B) N2
459
358
201
142
C=C
602
C=O
799
C) O2
C=C
835
C=0
1072
Multiple
Bonds
615
D) HCN
C=N
O=0
494
C=N
887
N=N
942
**All values in kJ/mol**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax