Which statement(s) is(are) true regarding the emission spectrum below? Select all that apply. n =00 n = 6 n = 5 -n = 4 Ln = 3 -n = 2 Line 4 Line 5 Line 1 _n = 1 Line 2 Line 3 The frequency associated with line 5 is greater than that associated with line 4. O Lines 1 and 3 represent emission and lines 2, 4, and 5 represent absorption. O The line associated with the longest wavelength is line 2. O The wavelength associated with line 3 is shorter than that associated with line 1. The photon of light associated with line 4 is more energetic than that associated with line 1. Energy
Which statement(s) is(are) true regarding the emission spectrum below? Select all that apply. n =00 n = 6 n = 5 -n = 4 Ln = 3 -n = 2 Line 4 Line 5 Line 1 _n = 1 Line 2 Line 3 The frequency associated with line 5 is greater than that associated with line 4. O Lines 1 and 3 represent emission and lines 2, 4, and 5 represent absorption. O The line associated with the longest wavelength is line 2. O The wavelength associated with line 3 is shorter than that associated with line 1. The photon of light associated with line 4 is more energetic than that associated with line 1. Energy
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 13PS: The most prominent line in the spectrum of mercury is at 253.652 nm. Other lines are located at...
Related questions
Question
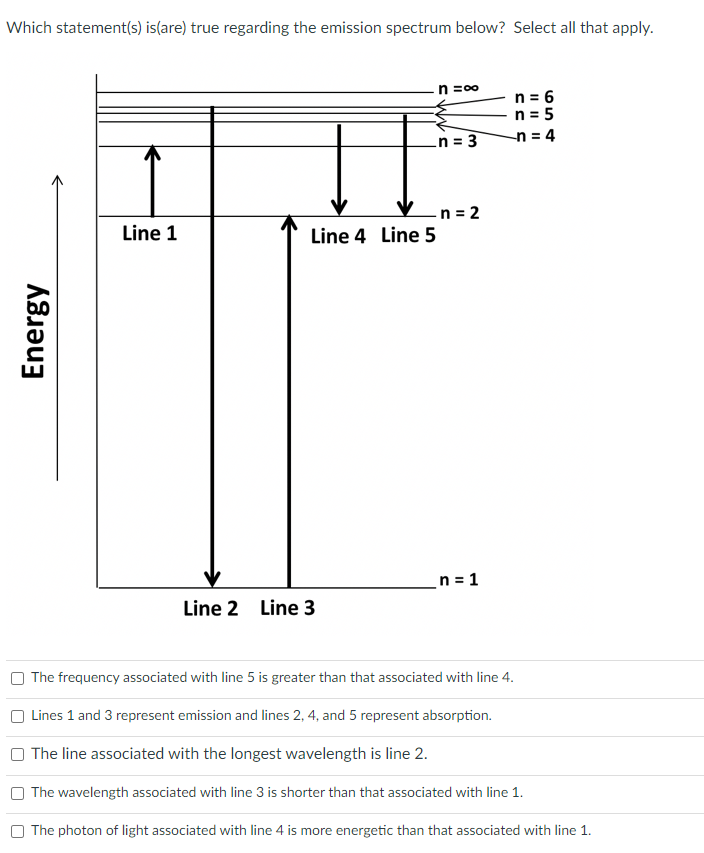
Transcribed Image Text:Which statement(s) is(are) true regarding the emission spectrum below? Select all that apply.
n =00
n = 6
n = 5
-n = 4
n = 3
-n = 2
Line 4 Line 5
Line 1
n = 1
Line 2 Line 3
The frequency associated with line 5 is greater than that associated with line 4.
Lines 1 and 3 represent emission and lines 2, 4, and 5 represent absorption.
The line associated with the longest wavelength is line 2.
The wavelength associated with line 3 is shorter than that associated with line 1.
The photon of light associated with line 4 is more energetic than that associated with line 1.
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning