Name: Ch. 2 Challenge Problem Set no anotong blos from owad 01 to Due Date: Sep. 22th @ 8:35am (turned in in person at the start of class). If you need an extension, you must ask for one via email by Sep. 20th. Otherwise, no late problem sets will be accepted. 1. Phakellin (3), a natural product isolated from marine organisms, has been studied for its potential use as an antibiotic agent. During studies aimed at developing a strategy for the synthesis of phakellin and its derivatives, compound 1 was investigated as a potential precursor. se gnu. KAW Mancilor9130 and oang Li and 1 N. erity (LDA) 2 is elving H₂N- b) Draw a curved arrow mechanism for the conversion of 1 to 2. goize bus ebrow N 3 a) Identify the most acidic proton in compound 1, draw the corresponding conjugate base, 2, and justify your choice. snowte) sinpr 3ROM 2Hno of ghidzug wone auturite plyort (s embra all now noitus94 noong dalawfd nsnotat eldsn02691 010112 500670291
Name: Ch. 2 Challenge Problem Set no anotong blos from owad 01 to Due Date: Sep. 22th @ 8:35am (turned in in person at the start of class). If you need an extension, you must ask for one via email by Sep. 20th. Otherwise, no late problem sets will be accepted. 1. Phakellin (3), a natural product isolated from marine organisms, has been studied for its potential use as an antibiotic agent. During studies aimed at developing a strategy for the synthesis of phakellin and its derivatives, compound 1 was investigated as a potential precursor. se gnu. KAW Mancilor9130 and oang Li and 1 N. erity (LDA) 2 is elving H₂N- b) Draw a curved arrow mechanism for the conversion of 1 to 2. goize bus ebrow N 3 a) Identify the most acidic proton in compound 1, draw the corresponding conjugate base, 2, and justify your choice. snowte) sinpr 3ROM 2Hno of ghidzug wone auturite plyort (s embra all now noitus94 noong dalawfd nsnotat eldsn02691 010112 500670291
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.39P: E. J. Coreys 1964 total synthesis of -caryophyllene (essence of cloves) solves a number of problems...
Related questions
Question
For #1a and #1b. I am having a difficult time identifying the most acidic proton.
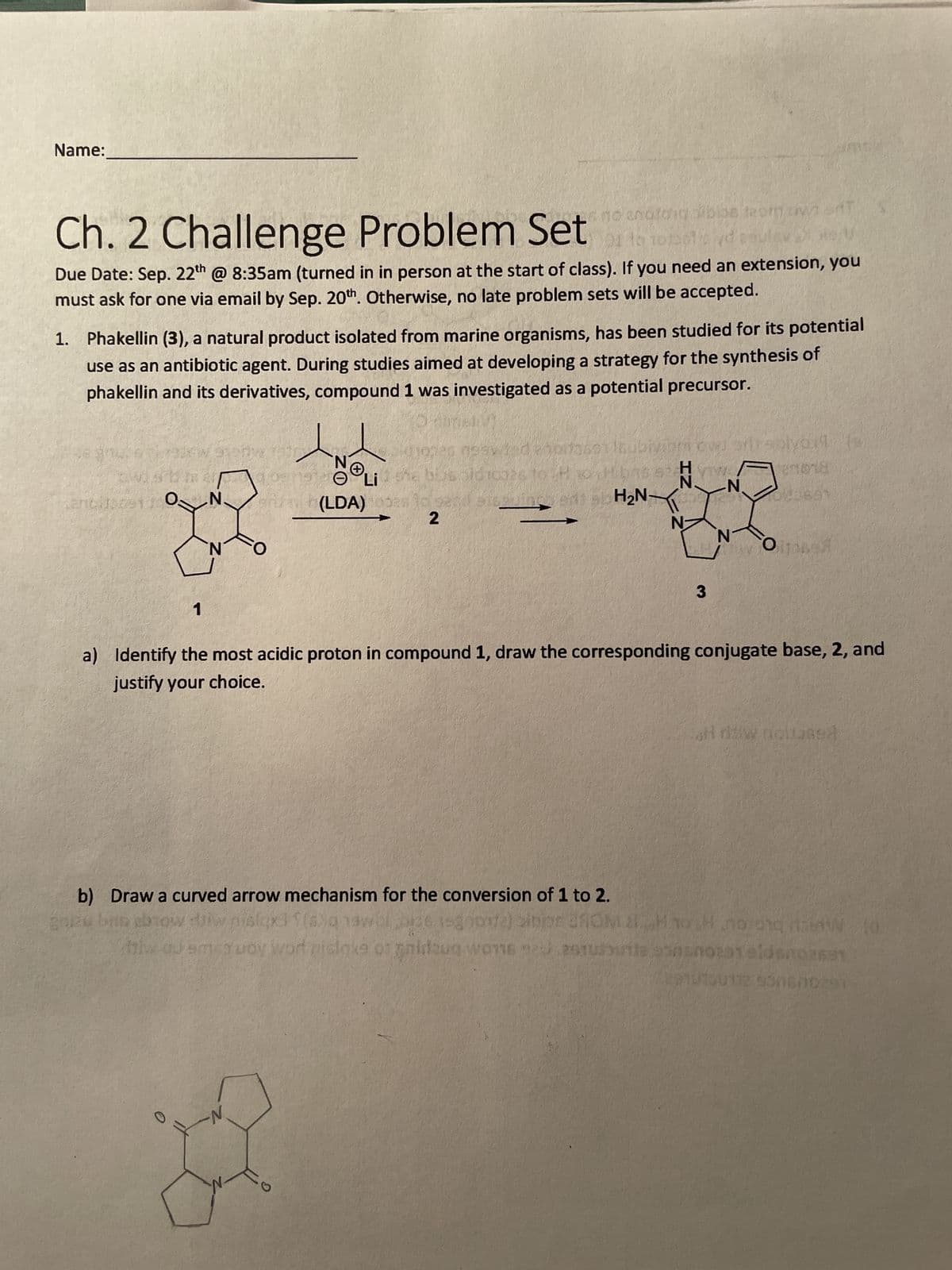
Transcribed Image Text:Name:
Ch. 2 Challenge Problem Setor torets d ample
sible from W
2
Due Date: Sep. 22th @ 8:35am (turned in in person at the start of class). If you need an extension, you
must ask for one via email by Sep. 20th. Otherwise, no late problem sets will be accepted.
1. Phakellin (3), a natural product isolated from marine organisms, has been studied for its potential
use as an antibiotic agent. During studies aimed at developing a strategy for the synthesis of
phakellin and its derivatives, compound 1 was investigated as a potential precursor.
grate their wor
CANA
mah
N.
1
N
0
1974 neavited epit7601
Libid 10926 to 26
10028
inc
N
aruah (LDA)
(LDA)
2
G
H₂N-
IZ
N
prirsplvert
27018
0801
670
N₁.
3
a) Identify the most acidic proton in compound 1, draw the corresponding conjugate base, 2, and
justify your choice.
N
How itonen
b) Draw a curved arrow mechanism for the conversion of 1 to 2.
av
new bus abrow diw nislgx3?(s) 1awel 36 1-gene) sinjor 3M 21H0H noronq AW to
miw ou amen woy word piclaxe or gnidzug wons 920 251uurite sono231 eldsn02691
2910730112 930670291
H daw nollased
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Why couldn't it be the H on the other side? Is it specific that only that one H can be the most acidic? Or does it not really matter because orienting the whole molecule such that we are looking at the opposite side of it, it is the same?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning