Chapter30: Kinetic Methods Of Analysis
Section: Chapter Questions
Problem 30.12QAP
Related questions
Question
Why did the 1st order integrated rate law allow you to find k from the slope?
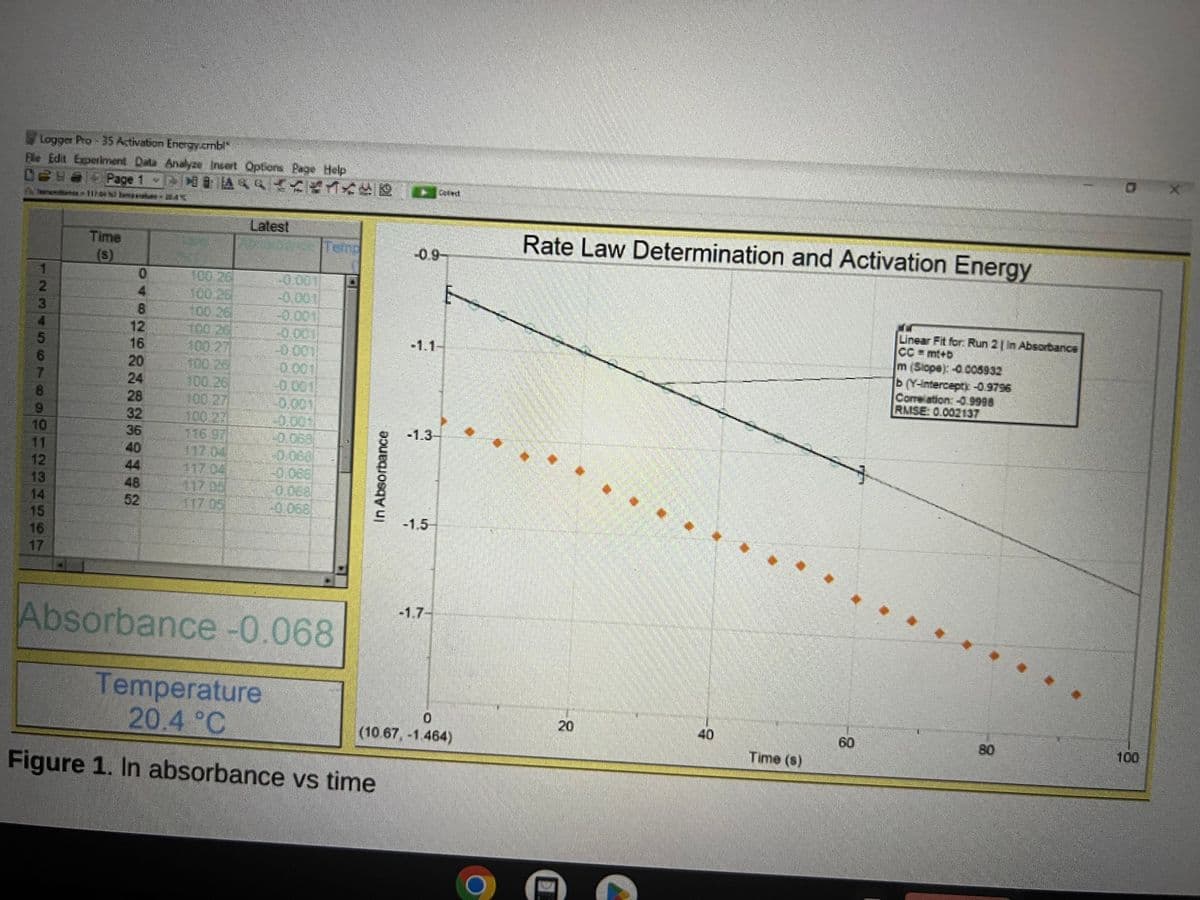
Transcribed Image Text:Logger Pro-35 Activation Energy.cmbl
Ele Edit Experiment Data Analyze Insert Options Page Help
Page 1 TO DO
1
2
3
4
5
6
7
8
9
10
11
13
14
15
16
Time
(s)
0
4
8
12
16
20
24
32
36
40
48
52
100.26
100.21
116.97
117.04
Latest
200010
Absorbance -0.068
In Absorbance
Temperature
20.4 °C
Figure 1. In absorbance vs time
-1.1-
-1.3
-1.5
-1.7-
E
0
(10.67, -1.464)
Rate Law Determination and Activation Energy
20
40
Time (s)
60
Linear Fit for: Run 2 | In Absorbance
CC=mt+b
m (Slope): -0.005932
b (Y-intercepty -0.9796
Correlation: -0.9998
RMSE 0.002137
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

