Why is methanol chosen as the solvent for recrystallization? (Check all that apply.) Because the product and the impurities dissolve well at the boiling point of the solvent. Because it can easily be boiled off. Because the reactants dissolves less than the product in methanol at low temperatures. Because the product dissolves less than the reactants in methanol at low temperatures. Because it has a low boiling point
Why is methanol chosen as the solvent for recrystallization? (Check all that apply.) Because the product and the impurities dissolve well at the boiling point of the solvent. Because it can easily be boiled off. Because the reactants dissolves less than the product in methanol at low temperatures. Because the product dissolves less than the reactants in methanol at low temperatures. Because it has a low boiling point
Chapter15: Benzene And Aromaticity
Section15.SE: Something Extra
Problem 40AP
Related questions
Question

Transcribed Image Text:Why is methanol chosen as the solvent for recrystallization?
(Check all that apply.)
Because the product and the impurities dissolve well at the
boiling point of the solvent.
Because it can easily be boiled off.
Because the reactants dissolves less than the product in
methanol at low temperatures.
Because the product dissolves less than the reactants in
methanol at low temperatures.
Because it has a low boiling point
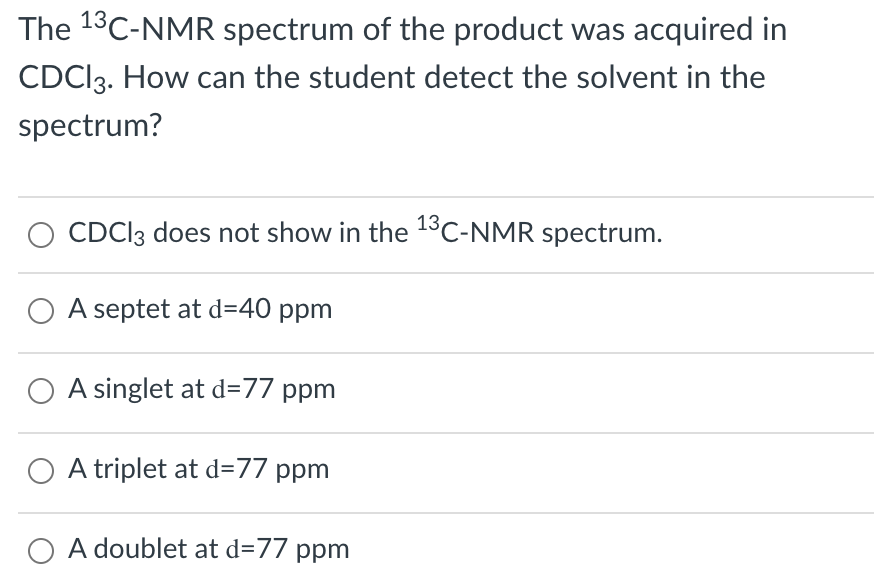
Transcribed Image Text:The 13C-NMR spectrum of the product was acquired in
CDCI3. How can the student detect the solvent in the
spectrum?
13
CDCI3 does not show in the C-NMR spectrum.
O A septet at d=40 ppm
A singlet at d=77 ppm
O A triplet at d=77 ppm
O A doublet at d=77 ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole