
Concept explainers
A compound of unknown structure gave the following spectroscopic data:
Mass spectrum: M+=88.1
IR: 3600 cm-1
1ΗNMR: 1.4 δ (2 H, quartet, J=7 Hz); 1.2 δ (6H, singlet): 1.0 δ (1 H, singlet); 0.9 δ (3 H, triplet, J=7 Hz)
13CNMR: 74, 35, 27, 25 δ
(a) Assuming that the compound contains C and H but may or may not contain O, give three possible molecular formulas
(b) How many protons (H) does the compound contain?
(c) What functional groups(s) does the compound contain?
(d) How many carbons does the compound contain?
(e) What is the molecular formula of the compound?
(f) What is the structure of the compound?
(g) Assign peaks in the molecule’s 1HNMR spectrum corresponding to specific protons.
a) Three molecular formulas possible for the compound are to be given assuming that the compound contains C and H but may or may not contain oxygen.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600 cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
a) Three molecular formulas possible for the compound assuming that the compound contains C and H but may or may not contain oxygen.
Answer to Problem 55AP
a) Three molecular formulas possible for the compound assuming that the compound contains C and H and O are C5H12O, C4H8O2 and C3H4O3.
Explanation of Solution
a) The IR absorption at 3600cm-1 indicates the presence of an alcoholic group in the compound. Hence assuming the compound contains C, H and O, three possible formulas are possible for the compound with molecular mass 88. They are C5H12O, C4H8O2 and C3H4O3.
Three molecular formulas possible for the compound assuming that the compound contains C and H but may or may not contain oxygen are C5H12O, C4H8O2 and C3H4O3.
b) The number of protons in the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600 cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
b) The number of protons in the compound.
Answer to Problem 55AP
b) The compound has 12 protons.
Explanation of Solution
b) IN 1HNMR spectrum contain peaks accounting for the absorption for 12 protons in the molecule.
b) The compound has 12 protons.
c) The functional group(s) present in the compound is/are to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
c) The functional group(s) present in the compound.
Answer to Problem 55AP
c) The functional group in the compound is –OH (alcohol).
Explanation of Solution
c) In 13HNMR only four signals are observed. But 12 protons could not be accommodated on four carbons. At least five carbons are required. This justified by the 1HNMR spectrum which an absorption integrating to six protons present on two equivalent carbons. Hence five carbons are present in the molecule.
c) The functional group in the compound is –OH (alcohol).
d) The number of carbons in the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
d) The number of carbons in the compound.
Answer to Problem 55AP
d) The compound has 5 carbons.
Explanation of Solution
d) Its molecular formula has to be C5H12O as it is a five carbon alcohol with 12 hydrogen atoms.
d) The compound has 5 carbons.
e) Molecular formula of the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
e) Molecular formula of the compound.
Answer to Problem 55AP
e) Molecular formula of the compound is C5H12O.
Explanation of Solution
e) The six proton singlet at 1.2 δ can be attributed to two methyl groups attached to a carbon without any proton. The two proton triplet at 1.4 δ can be assigned to CH2 attached to a methyl which gives a triplet at 0.9 δ. The one proton singlet at 1.0 δ is due to the hydroxyl proton.
e) Molecular formula of the compound is C5H12O.
f) The structure of the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
f) The structure of the compound.
Answer to Problem 55AP
f) The structure of the compound is
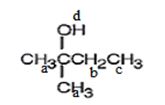
Explanation of Solution
f) Thus the structure of the alcohol is

The structure of the compound is

g) The peaks in the 1HNMR of the molecule are to be assigned to specific protons.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To assign:
g) The peaks in the 1HNMR of the molecule to specific protons.
Answer to Problem 55AP
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
Explanation of Solution
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry
- Compound A, a hydrocarbon with M+=96 in its mass spectrum, has the 13C spectral data given below. On reaction with BH3, followed by treatment with basic H2O2. A is converted into B, whose, 13C spectral data are also given below. Propose structures for A and B. Compound A Broadband-decoupled l3C NMR: 26.8, 28.7, 35.7, 106.9, 149.7 DEPT-90: no peaks DEPT-135: no positive peaks; negative peaks at 26.8, 28.7, 35.7, 106.9 Compound B Broadband-decoupled 13C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 DEPT-90: 40.5 DEPT-135: positive peak at 40.5 ; negative peaks at 26.1, 26.9. 29.9, 68.2arrow_forwardAddition of m-xylene to the strongly acidic solvent HF/SbF5 at 45C gives a new species, which shows 1H-NMR resonances at 2.88 (3H), 3.00 (3H), 4.67 (2H), 7.93 (1H), 7.83 (1H), and 8.68 (1H). Assign a structure to the species giving this spectrum.arrow_forwardPropose structures for compounds that fit the following mass-spectral data: (a) A hydrocarbon with M+=132 (b) A hydrocarbon with M+=166 (c) A hydrocarbon with M+=84arrow_forward
- Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardDetermine tthe structure of C9H10O from the 1H-NMR and IR spectraarrow_forward
- An aromatic compound K, whose molecular formula is C8H11N, is examined in the laboratory to elucidate its structure. The following observations were made: A) Compound K is soluble in dilute hydrochloric acid but insoluble in sodium hydroxide solution. B) Treatment of compound K with excess potassium hydroxide and benzenesulfonyl chloride, C(6)H(5)SO(2)Cl, results in the formation of a heterogeneous mixture. The NMR spectrum of compound K is shown below. C) Compound K when treated with acetic anhydride[CH3-C(O)-O-C(O)-CH3], gives compound L, whose molecular formula is C(10)H(13)ON. Compound L is insoluble in dilute acid or dilute base at room temperature, heating compound L in dilute acid or base, however, regenerates compound K. D) When compound L is heated with a mixture of concentrated nitric acid and sulfuric acid, a single product, compound M, with the molecular formula C(10)H(12)O(3)N(2) is formed in excellent yields. On the basis of these observations draw the structures of…arrow_forwardIdentify the structures of isomers H and I (molecular formula C8H11N).a.Compound H: IR absorptions at 3365, 3284, 3026, 2932, 1603, and 1497 cm−1b.Compound I: IR absorptions at 3367, 3286, 3027, 2962, 1604, and 1492 cm−1arrow_forwardPropose a structure for a compound of molecular formula C7H14O2 with an IR absorption at 1740 cm−1 and the following 1H NMR data: Absorption ppm Relative area singlet 1.2 9 triplet 1.3 3 quartet 4.1 2arrow_forward
- Treatment of benzoic acid (C6H5CO2H) with NaOH followed by 1-iodo-3methylbutane forms H. H has a molecular ion at 192 and IR absorptions at 3064, 3035, 2960−2872, and 1721 cm−1. Propose a structure for H.arrow_forwardDeduce the structures of compounds A and B, two of the major components of jasmine oil, from the given data. Compound A: C9H10O2; IR absorptions at 3091–2895 and 1743 cm-1; 1H NMR signals at 2.06 (singlet, 3 H), 5.08 (singlet, 2 H), and 7.33 (broad singlet, 5 H) ppm. Compound B: C14H12O2; IR absorptions at 3091–2953 and 1718 cm-1; 1H NMR signals at 5.35 (singlet, 2 H) and 7.26–8.15 (multiplets, 10 H) ppm.arrow_forwardPropose a structure for the compound that fits the following description. C10H14 7.0 δ, (4H, broad singlet) 2.85 δ (1H, septet J = 8 Hz) 1.20 δ (6H, doublet J = 8 Hz) 2.28 δ (3H, singlet) IR: 825 cm-1 5(a) Degree of the unsaturation of this compound is= , 5(b) Two peaks at 2.85 δ and 1.20 δ indicate that the compound has .......... group = 4(c) A peak at 2.28 δ indicates that the compound has ............. group= 4(d) IR absorption indicates the compound is .........-disubstituted = 4(e) The name of the compound is =arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

