Electron Transport Chain
The electron transport chain, also known as the electron transport system, is a group of proteins that transfer electrons through a membrane within mitochondria to create a gradient of protons that drives adenosine triphosphate (ATP)synthesis. The cell uses ATP as an energy source for metabolic processes and cellular functions. ETC involves series of reactions that convert redox energy from NADH (nicotinamide adenine dinucleotide (NAD) + hydrogen (H)) and FADH2(flavin adenine dinucleotide (FAD)) oxidation into proton-motive force(PMF), which is then used to synthesize ATP through conformational changes in the ATP synthase complex, a process known as oxidative phosphorylation.
Metabolism
Picture a campfire. It keeps the body warm on a cold night and provides light. To ensure that the fire keeps burning, fuel needs to be added(pieces of wood in this case). When a small piece is added, the fire burns bright for a bit and then dies down unless more wood is added. But, if too many pieces are placed at a time, the fire escalates and burns for a longer time, without actually burning away all the pieces that have been added. Many of them, especially the larger chunks or damp pieces, remain unburnt.
Cellular Respiration
Cellular respiration is the cellular process involved in the generation of adenosine triphosphate (ATP) molecules from the organic nutritional source obtained from the diet. It is a universal process observed in all types of life forms. The glucose (chemical formula C6H12O6) molecules are the preferred raw material for cell respiration as it possesses a simple structure and is highly efficient in nature.
need help with these 2 questions please, thank you!
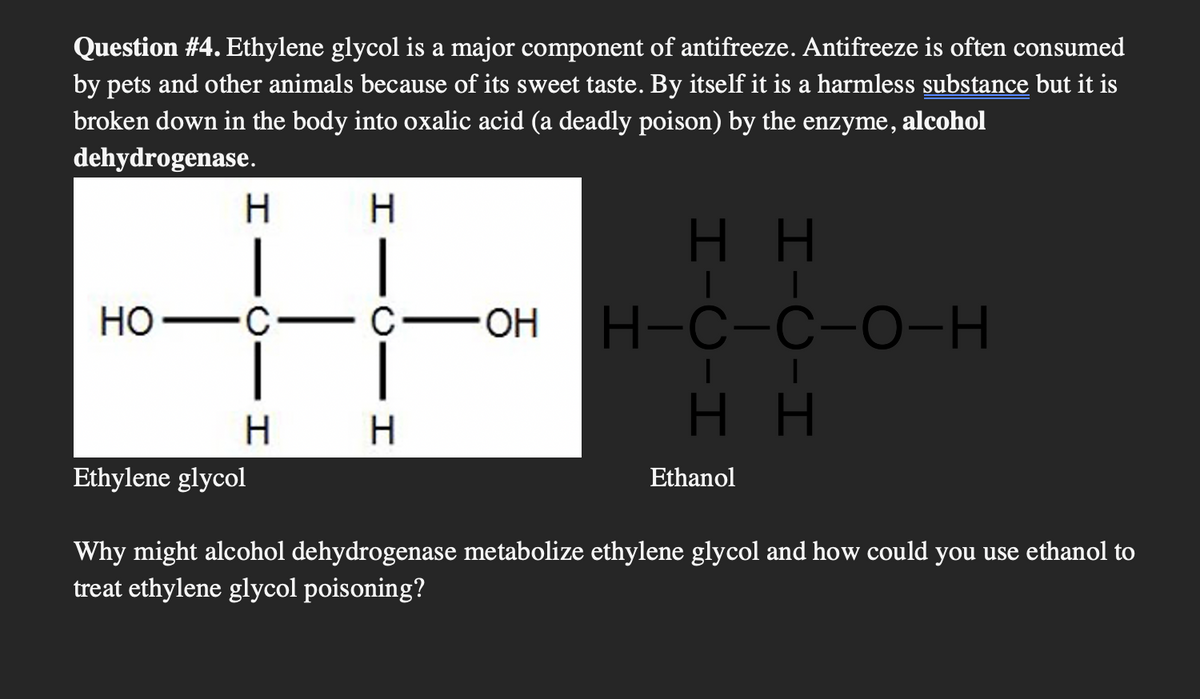
Ethylene glycol and ethanol have a very similar structure (only one OH group difference). Because of it's structural similarity to ethanol, ethylene glycol can bind to alcohol dehydrogenase and gets hydrolyzed by the said enzyme.
Step by step
Solved in 2 steps




