There are parts A-C for this picture included. A) What type of enzyme is Malate Dehydrogenase? choices: Hydrolase, Isomerase, Ligase, Oxideoreductase, Transferase, or Translocase B) Which of the following statements are true in biochemical standard conditions? There can be more than 1. Choices: The reaction is spontaneous since ∆G°' is positive The reaction is spontaneous since ∆G°' is negative The reaction is not spontaneous since ∆G°' is positive The reaction is not spontaneous since ∆G°' is positive The equilibrium favors products since K is greater than 1 The equilibrium favors reactants since K is greater than 1 The equilibrium favors products since K is less than 1 The equilibrium favors reactants since K is less than 1 The reaction is always at equilibrium C) If the concentration of Oxaloacetate is 10^7 times lower than the concentration of Malate D.,Is the reaction Spontanuous? Choices: No, because RTInQ is very positive Yes, because RTlnQ is very positive No, because RTlnQ is very negative Yes, because RTlnQ is very negative No, because RTlnQ is equal to ∆G°' Not enough information
There are parts A-C for this picture included. A) What type of enzyme is Malate Dehydrogenase? choices: Hydrolase, Isomerase, Ligase, Oxideoreductase, Transferase, or Translocase B) Which of the following statements are true in biochemical standard conditions? There can be more than 1. Choices: The reaction is spontaneous since ∆G°' is positive The reaction is spontaneous since ∆G°' is negative The reaction is not spontaneous since ∆G°' is positive The reaction is not spontaneous since ∆G°' is positive The equilibrium favors products since K is greater than 1 The equilibrium favors reactants since K is greater than 1 The equilibrium favors products since K is less than 1 The equilibrium favors reactants since K is less than 1 The reaction is always at equilibrium C) If the concentration of Oxaloacetate is 10^7 times lower than the concentration of Malate D.,Is the reaction Spontanuous? Choices: No, because RTInQ is very positive Yes, because RTlnQ is very positive No, because RTlnQ is very negative Yes, because RTlnQ is very negative No, because RTlnQ is equal to ∆G°' Not enough information
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
There are parts A-C for this picture included.
A) What type of enzyme is Malate Dehydrogenase?
choices: Hydrolase, Isomerase, Ligase, Oxideoreductase, Transferase, or Translocase
B) Which of the following statements are true in biochemical standard conditions? There can be more than 1. Choices:
The reaction is spontaneous since ∆G°' is positive
The reaction is spontaneous since ∆G°' is negative
The reaction is not spontaneous since ∆G°' is positive
The reaction is not spontaneous since ∆G°' is positive
The equilibrium favors products since K is greater than 1
The equilibrium favors reactants since K is greater than 1
The equilibrium favors products since K is less than 1
The equilibrium favors reactants since K is less than 1
The reaction is always at equilibrium
C) If the concentration of Oxaloacetate is 10^7 times lower than the concentration of Malate D.,Is the reaction Spontanuous? Choices:
No, because RTInQ is very positive
Yes, because RTlnQ is very positive
No, because RTlnQ is very negative
Yes, because RTlnQ is very negative
No, because RTlnQ is equal to ∆G°'
Not enough information
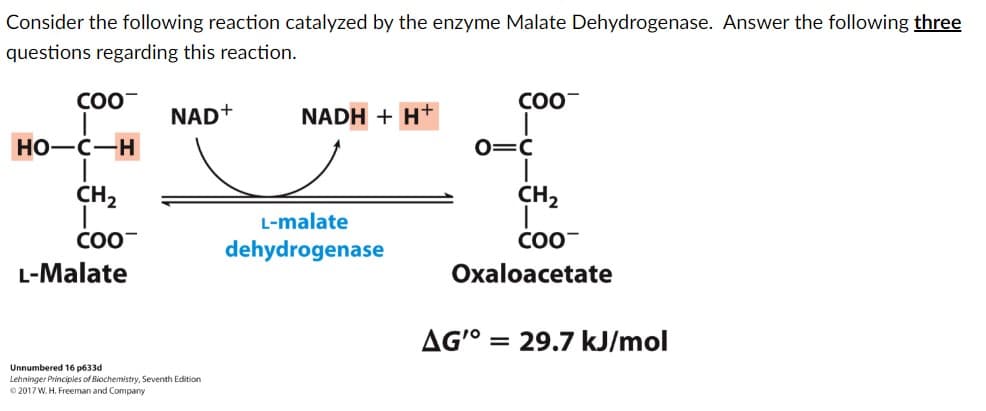
Transcribed Image Text:Consider the following reaction catalyzed by the enzyme Malate Dehydrogenase. Answer the following three
questions regarding this reaction.
COO
HO-C-H
CH₂
COO™
L-Malate
NAD+
Unnumbered 16 p633d
Lehninger Principles of Biochemistry, Seventh Edition
© 2017 W. H. Freeman and Company
NADH + H+
L-malate
dehydrogenase
COO™
O=C
CH₂
COO™
Oxaloacetate
AG'° = 29.7 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON