Williamson Ether Synthesis: The ether prepared in this experiment is a methylphenoxyacetic acid, which is a phenolic (benzene ring attached to something is a phenyl group) ether that is prepared from p-methylphenol (p-cresol) and chloroacetic acid. What is the percent yield obtained? Also, can you write balanced equation. materials used: 4.5 g of potassium hydroxide 2.55 g of p-cresol 6.70 mL of a 50 % aqueous solution (g/mL) of a-chloroacetic acid Final Product characterization: 2.30 g of a pale white solid
Williamson Ether Synthesis: The ether prepared in this experiment is a methylphenoxyacetic acid, which is a phenolic (benzene ring attached to something is a phenyl group) ether that is prepared from p-methylphenol (p-cresol) and chloroacetic acid. What is the percent yield obtained? Also, can you write balanced equation. materials used: 4.5 g of potassium hydroxide 2.55 g of p-cresol 6.70 mL of a 50 % aqueous solution (g/mL) of a-chloroacetic acid Final Product characterization: 2.30 g of a pale white solid
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter17: Carboxylic Acids
Section: Chapter Questions
Problem 17.45P
Related questions
Question
100%
What is the percent yield obtained? Also, can you write balanced equation.
materials used:
-
4.5 g of potassium hydroxide
-
2.55 g of p-cresol
-
6.70 mL of a 50 % aqueous solution (g/mL) of a-chloroacetic acid
Final Product characterization:
-
2.30 g of a pale white solid
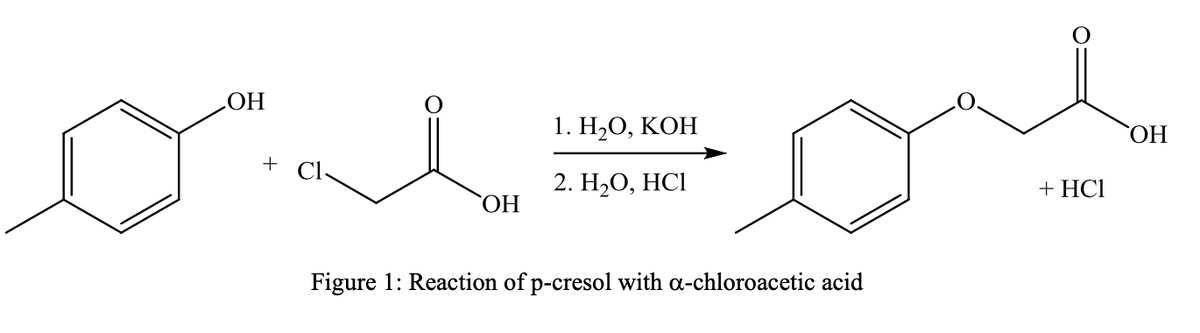
Transcribed Image Text:НО
1. Н,О, КОН
ОН
+
Cl
2. Н.О, НСІ
+ HC1
ОН
Figure 1: Reaction of p-cresol with a-chloroacetic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning