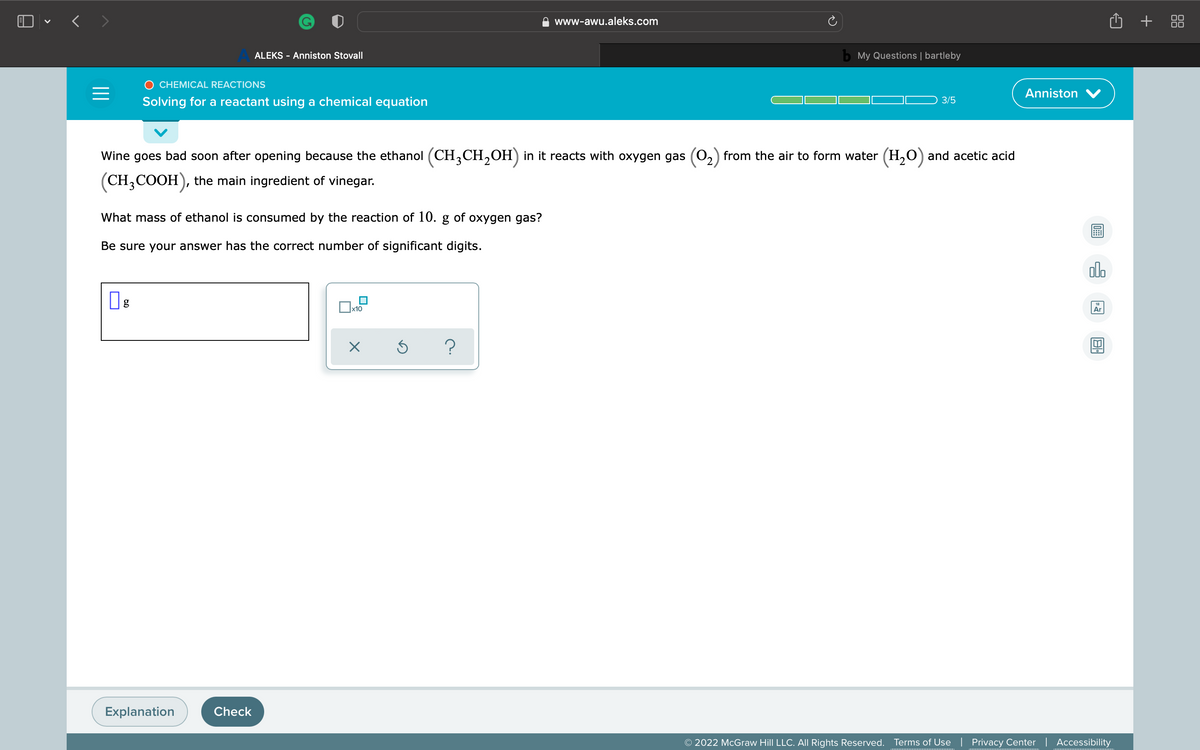
Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (02) from the air to form water (H,0) and acetic acid (CH;COOH), the main ingredient of vinegar. What mass of ethanol is consumed by the reaction of 10. g of oxygen gas? Be sure your answer has the correct number of significant digits.
Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (02) from the air to form water (H,0) and acetic acid (CH;COOH), the main ingredient of vinegar. What mass of ethanol is consumed by the reaction of 10. g of oxygen gas? Be sure your answer has the correct number of significant digits.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 16CR: The element carbon undergoes many inorganic reactions, as well as being the basis for the field of...
Related questions
Question
Transcribed Image Text:O • < >
+ 88
www-awu.aleks.com
ALEKS - Anniston Stovall
b My Questions | bartleby
O CHEMICAL REACTIONS
Anniston
Solving for a reactant using a chemical equation
3/5
Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the air to form water (H,O) and acetic acid
(CH,COOH), the main ingredient of vinegar.
What mass of ethanol is consumed by the reaction of 10. g of oxygen gas?
Be sure your answer has the correct number of significant digits.
olo
g
18
x10
Ar
Explanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved.
Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
