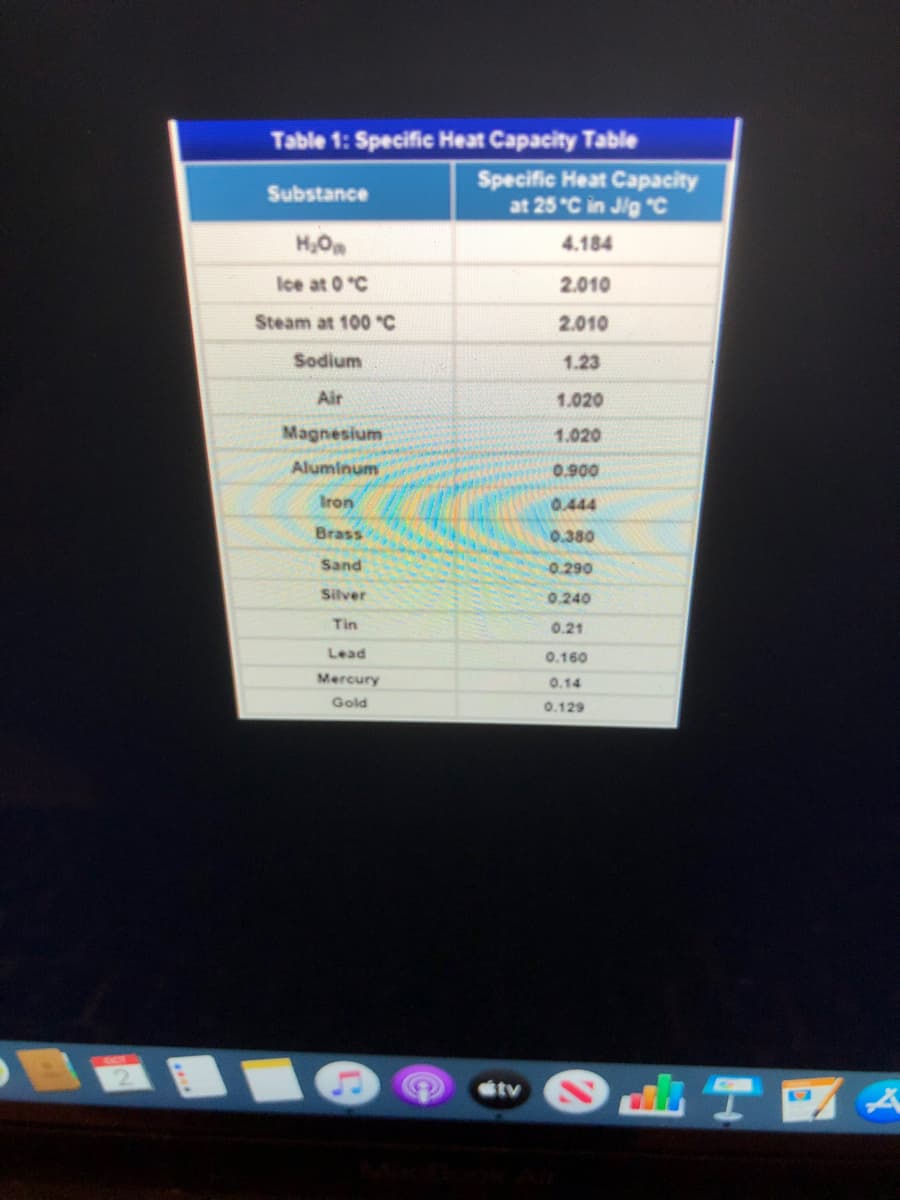
Write a CER. 15.6 grams of a 100°C piece of metal is placed in 110 grams of water. The water has a starting temperature of 22.5°C and an ending temperature of 23.5°C. Determine the specific heat of the metal. Then identify the metal using the table below. Water has a specific heat of 4.18 J/g°C.
Write a CER. 15.6 grams of a 100°C piece of metal is placed in 110 grams of water. The water has a starting temperature of 22.5°C and an ending temperature of 23.5°C. Determine the specific heat of the metal. Then identify the metal using the table below. Water has a specific heat of 4.18 J/g°C.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.47QP: A quantity of ice at 0C is added to 64.3 g of water in a glass at 55C. After the ice melted, the...
Related questions
Question
Explain how you know your claim is correct and how energy was transferred in this scenario only
Transcribed Image Text:Sublimation
O Melting
Exot
Deposition
Co
Fre
Dep
Write a CER. 15.6 grams of a 100°C piece of metal is placed in 110
grams of water. The water has a starting temperature of 22.5°C
and an ending temperature of 23.5°C. Determine the specific heat
of the metal. Then identify the metal using the table below. Water
has a specific heat of 4.18 J/g°C.
3\
Show your calculations below.
31,965
OCT
tv
80
=1
000
F2
F3
F4
F5
F6
Transcribed Image Text:Table 1: Specific Heat Capacity Table
Specific Heat Capacity
at 25 C in Jig "C
Substance
H,O
4.184
Ice at 0 C
2.010
Steam at 100 "C
2.010
Sodium
1.23
Air
1.020
Magnesium
1.020
Aluminum
0.900
Iron
0.444
Brass
0.380
Sand
0.290
Silver
0.240
Tin
0.21
Lead
0.160
Mercury
0.14
Gold
0.129
Tス
stv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER