Write balanced equations for the following reactions. Usestructural formulas to represent the organic compounds.(a) The complete combustion (to CO2 and H2O) of cyclopropanol(b) The reaction of isopropyl acetate with water to give acetic acid and isopropanol(c) The dehydration of ethanol to give ethene(d) The reaction of 1-iodobutane with water to give 1-butanol
Write balanced equations for the following reactions. Usestructural formulas to represent the organic compounds.(a) The complete combustion (to CO2 and H2O) of cyclopropanol(b) The reaction of isopropyl acetate with water to give acetic acid and isopropanol(c) The dehydration of ethanol to give ethene(d) The reaction of 1-iodobutane with water to give 1-butanol
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter21: Organic Chemistry
Section: Chapter Questions
Problem 21.4TC
Related questions
Question
Write balanced equations for the following reactions. Use
structural formulas to represent the organic compounds.
(a) The complete combustion (to CO2 and H2O) of
cyclopropanol
(b) The reaction of isopropyl acetate with water to give
acetic acid and isopropanol
(c) The dehydration of ethanol to give ethene
(d) The reaction of 1-iodobutane with water to give
1-butanol
Expert Solution
Step 1
(a)
The balanced chemical equation for the complete combustion of cyclopropanol is shown as:
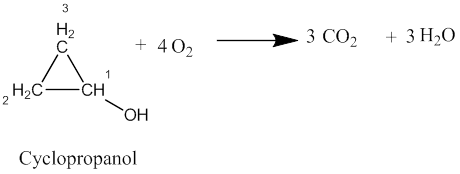
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning