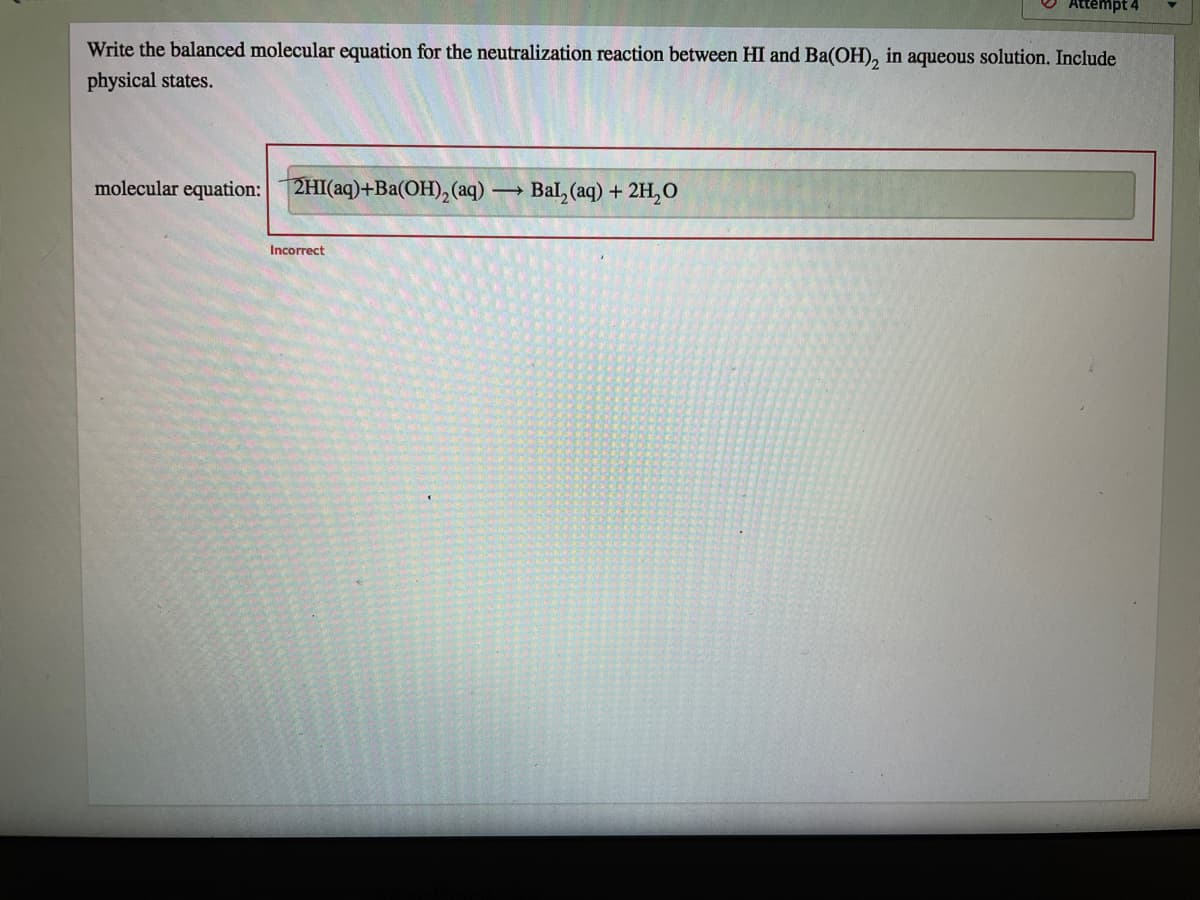
Write the balanced molecular equation for the neutralization reaction between HI and Ba(OH), in aqueous solution. Include physical states. molecular equation:2HI(aq)+Ba(OH), (aq) → Bal, (aq) + 2H,O Incorrect
Q: the following chemical reaction takes place in aqueous solution: What is the net ionic equation for…
A: Dear student I have given answer to your question in the image format.
Q: To measure the concentration of an aqueous solution of H,0,, an analytical chemist adds strong acid…
A: In a precipitation reaction, precipitation should appear. In a redox reaction oxidation and…
Q: What is the balanced molecular reation, total ionic reaction and net reatction of…
A: In a chemical reaction; the substance which involves in conversion is said to be reactant whereas…
Q: Predict the products for the following precipitation reactions. For each, write the balanced net…
A: Spectator ions are the ones that seems to remain unchanged in the overall reaction. Let's see the…
Q: rite the net ionic equation, including phases, that corresponds to the reaction Pb(CIO,),(aq) +…
A: Given : We have to write the net ionic equation.
Q: a What is the balanced molecular equation for the following reaction? HCIO4 + CaC03 → ited the…
A: • We need to write the balanced molecular equation for the reaction of HClO4 + CaCO3
Q: 13. (4.54 Brown) Write balanced molecular and net ionic equations for the reactions of a.…
A: Since you have posted questions with multiple sub-parts, we are entitled to answer the first 3 only.…
Q: Draw the major organic product of each of the following reactions.
A:
Q: Write the net ionic equation, including phases, that corresponds to the reaction Zn(CIO,),(aq) +…
A:
Q: Write the balanced NET ionic equation for the reaction when AICI3 and NaOH are mixed in aqueous…
A: The net ionic equation is the chemical equation that shows only those elements, compounds, and ions…
Q: A precipitation reaction occurs when the two solutions in separate test tubes are mixed: Test tube…
A: Oxidation is lose of electrons. Reduction is gaining of electrons. The ions which do not participate…
Q: Identify the following reactions as acid-base reactions (AB), precipitation reactions (PPT),…
A:
Q: Select the spectator ions for the following reaction in aqueous solution, AgNO3 + LiI → AgI +…
A: In a chemical reaction, spectator ions are ones that don't take part in the reaction or in other…
Q: Sometimes a reaction can fall in more than one category. Into what category (or categories) does the…
A: What type of reaction involve in the reaction given above---
Q: Write the net ionic equation for the reaction shown. Include physical states. 2 HNO, (aq)+Sr(OH),…
A: We have to predict the net ionic equation.
Q: Write the net ionic equation, including phases, that corresponds to the reaction Zn(NO, ),(aq) +…
A: We have to predict the net ionic equation.
Q: Some chemical reactants are listed in the table below. Complete the table by filling in the…
A: Oxidation number is defined as charge on an atom if all of its bonds to different atoms were fully…
Q: Complete and balance the molecular equation for the reaction of aqueous iron(III) nitrate,…
A: A balanced chemical equation is the one that consists of equal number of every atom of an element at…
Q: Complete and balance each of the following molecular equations involving acid-base reactions.…
A: Ans The given equation 5. Al(OH)3(aq)+ 3HCl(aq) ---> Reaction of the Al(OH)3(aq) with HCl(aq)…
Q: Write the balanced NET ionic equation for the reaction when HNO₃ and CsOH are mixed in aqueous…
A: A chemical equation in which electrolytes of an aqueous solution are represented in the form of…
Q: W the balanced complete lonic equations and net ionic equations for the reactions that occur when…
A: a) The reactants given are Pb(NO3)2 and Na2S.
Q: Write the net ionic equation for this precipitation reaction. Include physical states. 2…
A: The balanced chemical equation is: 2RbOHaq+MgNO32aq→Mg(OH)2s+2RbNO3aq
Q: a) Write the predicted products for the chemical reactions given below. (Note: If you consider that…
A: These problems can be solved with the aid of Electrochemical series (ECS) which is also known as…
Q: Nitrogen-fixing bacteria and plants are capable of converting atmospheric nitrogen to ammonia. This…
A: The conversion of atmospheric nitrogen to ammonia takes place as follows: It is a type of reduction…
Q: Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction…
A: Given;
Q: Write the balanced NET IONIC equation for the reaction that occurs when hydroiodic acid and…
A:
Q: What kind of reaction is this if you said this was a precipitation reaction wright down the chemical…
A: The given reaction is as follows:
Q: Write balanced (a) molecular, (b) overall ionic, and (c) net ionic equations for the reaction…
A: Aqueous solution of phosphoric acid reacts with the aqueous solution of sodium hydroxide to form…
Q: Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide…
A:
Q: Write the balanced molecular equation for the neutralization reaction between HI and Ba(OH), in…
A: The balance molecular equation for the neutralization reaction between HI & Ba(OH)2 = ?
Q: Write balanced molecular and net ionic equations for the reactionsof (a) manganese with dilute…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: CaO(s) + CO, (g) CACO, (s) O acid-base neutralization O precipitation O redox O none of these…
A: Since you asked multiple questions so as per Q&A guidelines of portal I solve first image…
Q: For the following double-replacement reaction: (NH4)2SO4(aq) + CaBr2(aq) a) Predict the products…
A: 1.) a.) To write the products , we would use given solubility rules and see if any solid product…
Q: Write a net ionic equation for the reaction that occurs when aqueous solutions of hydroiodic acid…
A: The reactants given are hydroiodic acid i.e HI and potassium hydroxide i.e KOH.
Q: 4.26 Write balanced net ionic equations for the reactions that oc- cur in each of the following…
A:
Q: 1. Write and balance the complete molecular and net ionic equations for the reaction of KHP with…
A:
Q: Predict if a reaction will occur when Sn(s) and HBr(aq) are combined. If a reaction occurs, write a…
A: The reaction between Sn and HBr is given as follows: Sns+HBraq→SnBr2aq+H2g
Q: Write the molecular equation for the neutralization of LIOH(ag) solution by aqueous perchloric acid.…
A: • We need to write the molecular equation for the reaction of aqueous lithium hydroxide and aqueous…
Q: Zinc hydroxide is insoluble in water but dissolves when a ni-tric acid solution is added. Why? Write…
A: The reaction of Zinc hydroxide with Nitric acid is as follows: Zn(OH)2 (s)+ 2HNO3 (aq)→Zn(NO3)2…
Q: Consider the following (unbalanced) net ionic equation: IO3-(aq) + I-(aq) → I2(s) 1. Identify the…
A: 1) The reaction given is, => IO3- (aq) + I- (aq) → I2 (s) Since IO3- is having O in -2 oxidation…
Q: The net ionic equation for the aqueous neutralization reac-tion between acetic acid and sodium…
A: Write a neutralization reaction between Acetic acid and sodium hydroxide. Hydrochloric acid and…
Q: 3. The double replacement between silver nitrate solution and sodium chloride solution is written…
A: Reversible reaction : The reaction which can proceed in both directions i.e. forward and backward.…
Q: Write the balanced NET ionic equation for the reaction when HI and Sr(OH), are mixed in aqueous…
A: According to the question Sr(OH)2 and HI reacts to give SrI2 and H2O. Molecular equation : Sr(OH)2…
Q: Enter the balanced complete ionic equation for the acid-base reaction HC2H3O2(aq)+LiOH(aq)→…
A: The reactants given are 1) Acid : HC2H3O2 (aq) 2) Base : LiOH (aq) Hence acid will react with base…
Q: Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide…
A: Please find your solution below : Ionic equation is an equation in which the ionic compounds in ghe…
Q: For the reaction, Mg (s) and FeCl3 (aq), write (a.) the molecular, (b.) complete ionic, and (c.) net…
A:
Q: Write the balanced molecular chemical equation for the reaction in aqueous solution for barium…
A: According to the solubility rule, sulfates of all the metals are soluble except BaSO4, CaSO4,…
Q: Write the balanced molecular chemical equation for the reaction in aqueous solution for copper(I)…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- What is the product of the following reaction equation after balancing? If no reaction state that " Mg(s)+HCI(aq) ---->Balance the following in acidic medium: MnO4- + Fe2+ ---> Mn2+ + Fe3+ How many Fe2+ are there in the balanced equation?What is the % purity of a sample of oxalic acid if a 0.4006g sample requires 28.62mL of potassium permanganate solution, of which 1mL contains 5.98mg potassium permanganate? A. Write the chemical reaction showing the OS of each elementB. Show the half-reactions and balance the number of electrons lost and gainedC. Identify the reducing agent and the oxidizing agent and solve for their milliequivalent weightsD. Write the balanced equilibrium reactionE. Show your solution for % purity; the answer is 85.2%
- A precipitate of AgCl + AgBr weighs 0.8132 g. On heating on a current of Cl2, the AgBr is converted to AgCl, the mixture loses 0.1450 g in wt. Find the percentage Cl in the original sample.Answer d to i:The purity of the salt Sn3(PO4)2 (546.0727), was determined by the redox titration with KMnO4. Half reactions involved:Unbalanced half reactions: MnO4- ↔ Mn2+Sn2+ ↔ Sn4+ a) Balance the redox reaction given above. b) Write the fundamental equation for the redox stoichiometry between Sn2+ and MnO4- Before analysis, KMnO4 solution was standardized according to the following procedure: 1.000 g of 100% pure tin, Sn (118.71) wire was used. After proper chemical treatment, a volume of 29.75 mL of the permanganate solution was used in the titration of the reduced tin. c) Calculate the concentration of the KMnO4 solution. An as received sample of the salt Sn3(PO4)2 (546.0727) weighing 5.0550 g was dissolved in water. The sample was treated to completely reduce all the tin to Sn2+. This solution was then titrated with standard KMnO4 (158.03) (calculated in (c)). 40.00 mL of the standard KMnO4 solution was used in the titration to reach the equivalence point. d) What is the purity…A precipitate of AgCl + AgBr weighs 0.8132 gram. On heating in a current of chlorine, the AgBr is converted into AgCl, the mixture losing 0.1450 gram in weight. What was the percentage of chlorine in the original precipitate?
- a. Balance the following redox in acidic media C2O42- (aq) + MnO4- (aq) → CO2(aq) + Mn2+ (aq) b. If you titrated this reaction to determine the moles of C2O42-, the colormetric endpoint is pink due to the Mn2+. How many moles of the oxalate (C2O42-) existed in solution if 12 mL of 0.791M permanganate was needed to hit the endpoint?Which substance functions as a reducing agent in the following reaction CH4+ 2O2-->CO2+ 2H2OWhat is the Molarity of a NaOH solution of 25.0 ml of this NaOH solution is required to neutralize 20.0 ml of 0.30 M H2SO4 Write Balance equation to acertaron the stoichiometry of this reaction? explain me balance equation please!
- If you complete and balance the following equation in acidicsolutionMn2+(aq) + NaBiO3(s)---->Bi3+(aq)+ MnO4-(aq) + Na+(aq)how many water molecules are there in the balanced equation(for the reaction balanced with the smallest whole-numbercoefficients)? (a) Four on the reactant side (b) Three on the product side (c) One on the reactant side (d) Seven on theproduct side (e) Two on the product sidebalanced molecular equation (ME) write the balanced complete ionic equation (CIE) and write the balanced net ionic equation (NIE) Tin(II) chloride + potassium nitrate SnCl2(aq) KNO3(aq) Observations: No observable change; solution mixture is clear and colorless ME: CIE: NIE:A solution of hydrobromic acid is added to a solution of sodium hydroxide. Which of the following is true about the reaction between HBr and NaOH? SELECT ALL THAT APPLY! A. NaBr(aq) is formed as a product. B. The net ionic equation is: H+(aq) + OH-(aq) --> H2O(l) C. NaBr is a solid precipitate that forms. D. H2O (l) is formed as a product.

