Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 40QAP: Atoms form ions so as to achieve electron configurations similar to those of the noble gases. For...
Related questions
Question
Transcribed Image Text:A ALEKS-Xzavian Patrick - Learn
← CO
ctri
shift ↑
tab
PROBOOK
#
caps lock
esc
Explanation
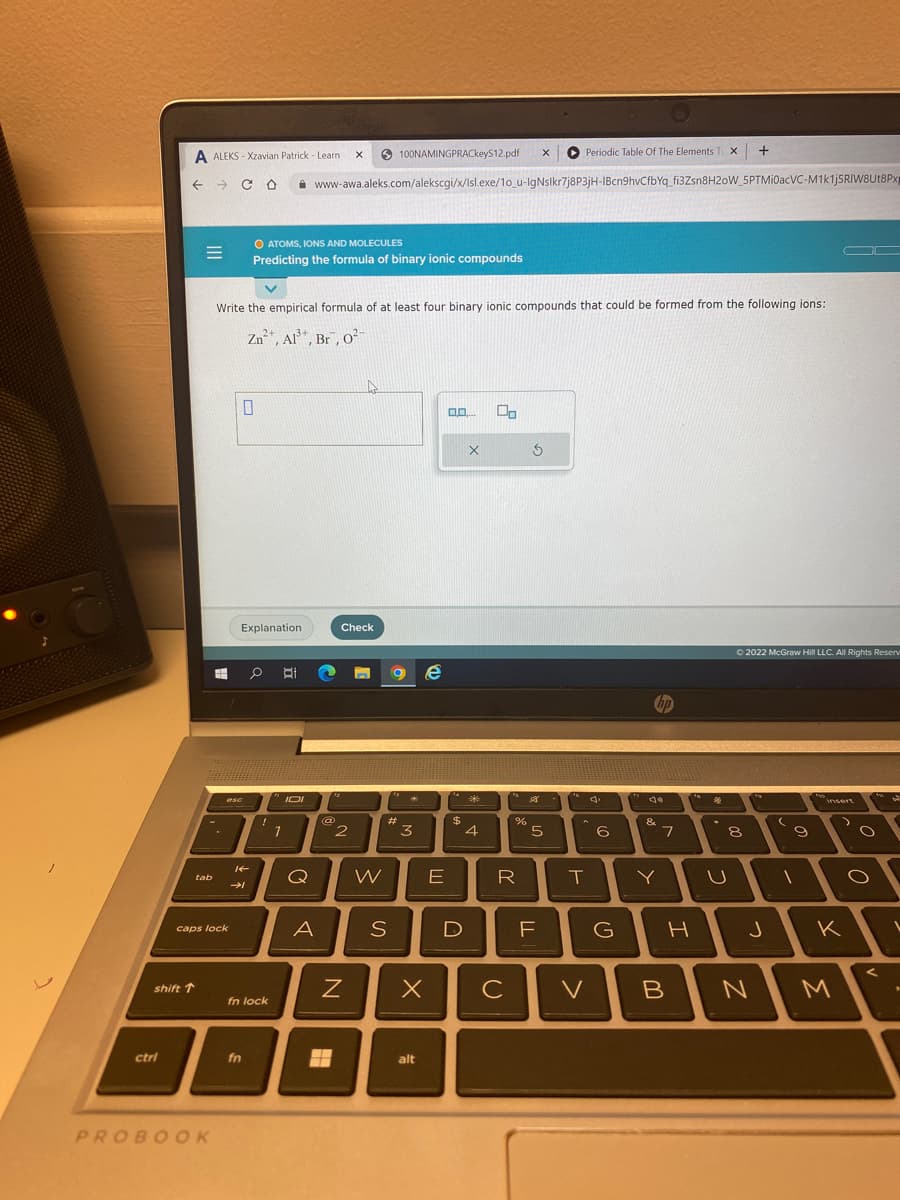
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions:
Zn²+, Al³+, Br, 0²
0
K
→1
fn
O ATOMS, IONS AND MOLECULES
Predicting the formula of binary ionic compounds
o af
!
fn lock
1
Periodic Table Of The Elements TX
www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H20W_5PTMi0acVC-M1k1j5RIW8Ut8Pxp
IOI
@
A
X
Check
2
8
Q W
N
100NAMINGPRACkey$12.pdf
n O e
#
S
*
3
E
x
alt
0.0...
$
D
X
**
4
с
2
%
R
X
A
S
5
F
to
A
T
4,
V
6
G
8
Y
7
H
B
#
.
+
8
U
Ⓒ2022 McGraw Hill LLC. All Rights Reserv
J
(
9
vo
C
K
N M
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning