Write the equilibrium constant expressions for K. and Kp, if applicable, for the following reactions: (a) 2NO2(8) + 7H2(8) = 2NH3(8) + 4H20(1) (b) 2ZnS(s) + 302(8) 2Zn0(s) + 2SO2(8) (c) C(s) + CO2(8) (d) C,HSCOOH(aq) = = 2C0(g) CH;CO0 (aq) + H* (aq)
Write the equilibrium constant expressions for K. and Kp, if applicable, for the following reactions: (a) 2NO2(8) + 7H2(8) = 2NH3(8) + 4H20(1) (b) 2ZnS(s) + 302(8) 2Zn0(s) + 2SO2(8) (c) C(s) + CO2(8) (d) C,HSCOOH(aq) = = 2C0(g) CH;CO0 (aq) + H* (aq)
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 6RQ: Distinguish between the terms equilibrium constant and reaction quotient. When Q = K, what does this...
Related questions
Question
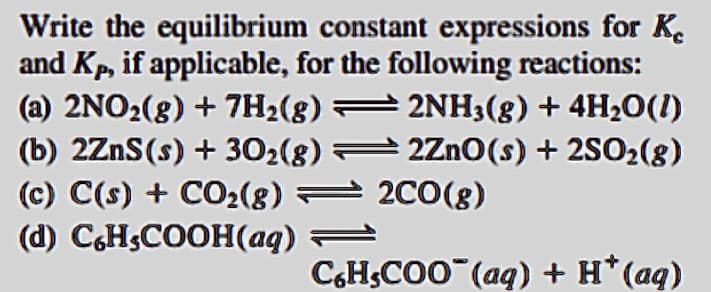
Transcribed Image Text:Write the equilibrium constant expressions for K.
and Kp, if applicable, for the following reactions:
(a) 2NO2(8) + 7H2(8) = 2NH3(g) + 4H20(1)
(b) 2ZnS(s) + 302(8) 2Zn0(s) + 2SO2(8)
(c) C(s) + CO2(8) = 2C0(8)
(d) C,HSCOOH(aq) =
CH;CO0 (aq) + H*(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax