Write the formula and then find the molar mass. e. iron(III) hydroxide f. tin(II) chloride a. sodium hydrogen carbonate b.cerium hexaboride C. magnesium perchlorate g. tetraphosphorus decoxide d. aluminum sulfate h. iodine monochloride
Write the formula and then find the molar mass. e. iron(III) hydroxide f. tin(II) chloride a. sodium hydrogen carbonate b.cerium hexaboride C. magnesium perchlorate g. tetraphosphorus decoxide d. aluminum sulfate h. iodine monochloride
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 48QAP: Calculate the percent by mass of the element listed first in the formulas for each of the following...
Related questions
Question
2. Write the formula and then find the molar mass.
a. sodium hydrogen carbonate
b. cerium hexaboride
c. magnesium perchlorate
d. aluminum sulfate
e. iron(III) hydroxide
f. tin(II) chloride
g. tetraphosphorus decoxide
h. iodine monochloride
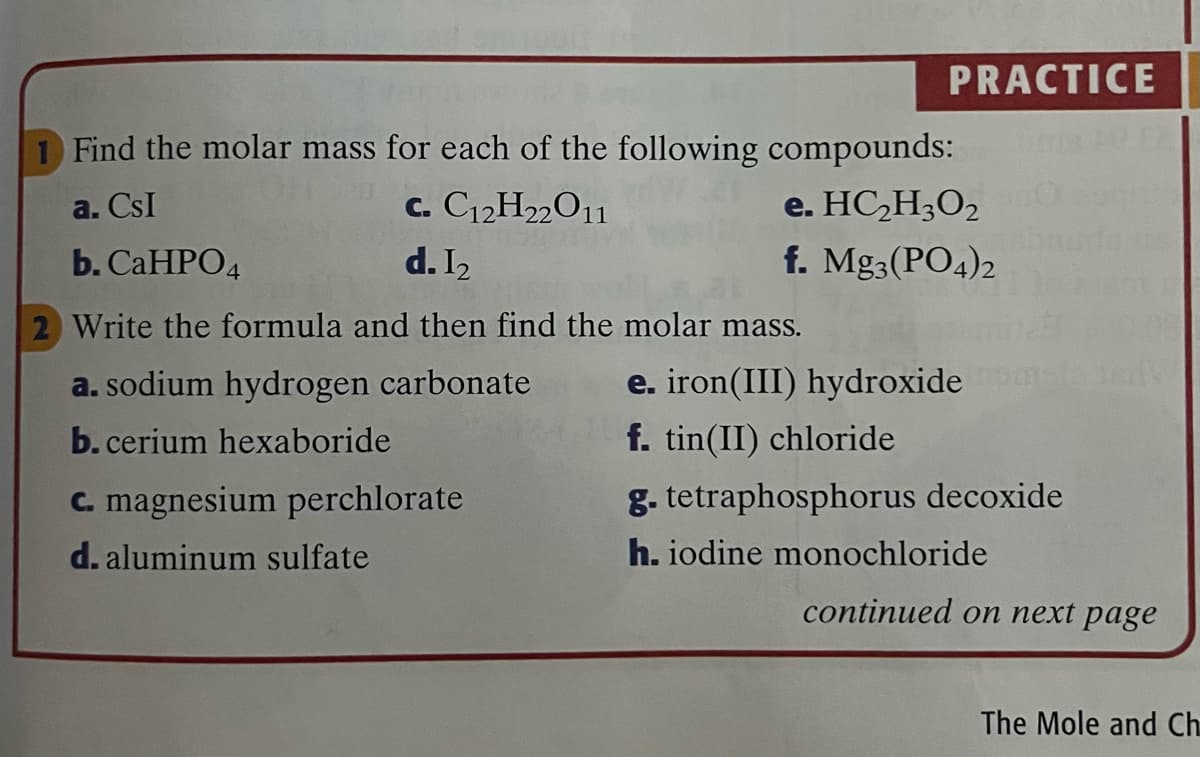
Transcribed Image Text:PRACTICE
Find the molar mass for each of the following compounds:
a. CsI
c. C12H22O1
e. HC,H3O2
b. CaHPО4
d. I2
f. Mg3(PO4)2
2 Write the formula and then find the molar mass.
a. sodium hydrogen carbonate
e. iron(III) hydroxide
b. cerium hexaboride
f. tin(II) chloride
C. magnesium perchlorate
g. tetraphosphorus decoxide
d. aluminum sulfate
h. iodine monochloride
continued on пеxt page
The Mole and Ch.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning