Write the half-reactions and macroscopic observations for copper and silver. Half-reactions should include 0 oxidation states if needed. Example of macroscopic observations is attached and half-reactions.
Write the half-reactions and macroscopic observations for copper and silver. Half-reactions should include 0 oxidation states if needed. Example of macroscopic observations is attached and half-reactions.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Write the half-reactions and macroscopic observations for copper and silver. Half-reactions should include 0 oxidation states if needed.
Example of macroscopic observations is attached and half-reactions.
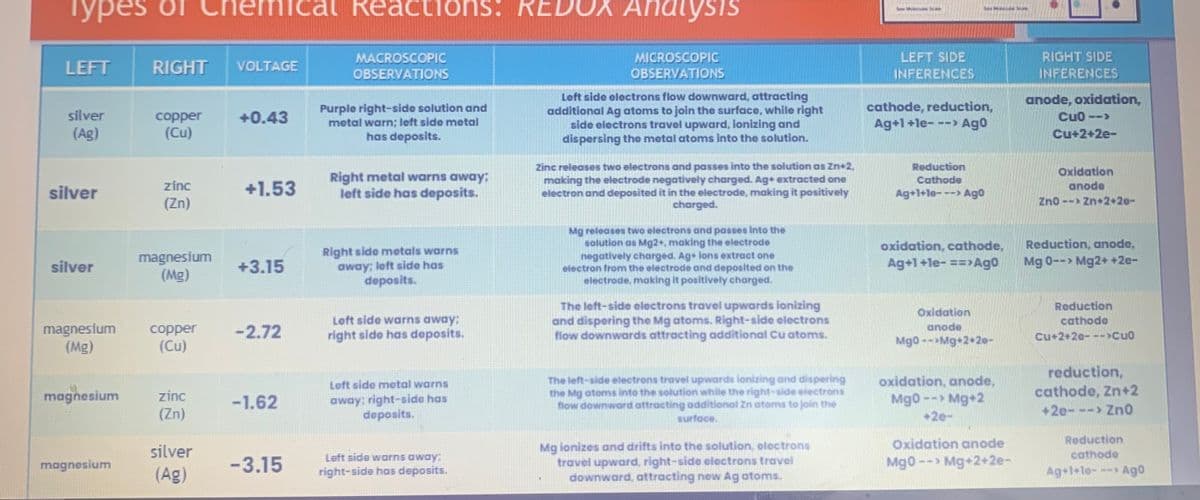
Transcribed Image Text:Types
LEFT
silver
(Ag)
silver
silver
magnesium
(Mg)
magnesium
magnesium
RIGHT
copper
(Cu)
zinc
(Zn)
magnesium
(Mg)
copper
(Cu)
zinc
(Zn)
silver
(Ag)
VOLTAGE
+0.43
+1.53
+3.15
-2.72
-1.62
-3.15
Reactions!
MACROSCOPIC
OBSERVATIONS
Purple right-side solution and
metal warn; left side metal
has deposits.
Right metal warns away;
left side has deposits.
Right side metals warns
away; left side has
deposits.
Left side warns away;
right side has deposits.
Left side metal warns
away: right-side has
deposits.
Left side warns away:
right-side has deposits.
Analysis
MICROSCOPIC
OBSERVATIONS
Left side electrons flow downward, attracting
additional Ag atoms to join the surface, while right
side electrons travel upward, ionizing and
dispersing the metal atoms into the solution.
zinc releases two electrons and passes into the solution as Zn+2,
making the electrode negatively charged. Ag+ extracted one
electron and deposited it in the electrode, making it positively
charged.
Mg releases two electrons and passes into the
solution as Mg2+, making the electrode
negatively charged. Ag+ lons extract one
electron from the electrode and deposited on the
electrode, making it positively charged.
The left-side electrons travel upwards ionizing
and dispering the Mg atoms. Right-side electrons
flow downwards attracting additional Cu atoms.
The left-side electrons travel upwards lonizing and dispering
the Mg atoms into the solution while the right-side electrons
flow downward attracting additional In atoms to join the
surface.
Mg ionizes and drifts into the solution, electrons
travel upward, right-side electrons travel
downward, attracting new Ag atoms.
M
LEFT SIDE
INFERENCES
See Mp
cathode, reduction,
Ag+1+le-
--> Ago
Reduction
Cathode
Ag+1+le---> Ago
oxidation, cathode,
Ag+1+le- ==> Ago
Oxidation
anode
Mg0Mg 2+20-
oxidation, anode,
Mg0 --> Mg+2
+26-
Oxidation anode
Mg0 --> Mg+2+2e-
RIGHT SIDE
INFERENCES
anode, oxidation,
CuO -->
Cu+2+2e-
Oxidation
anode
Zno--> Zn+2+20-
Reduction, anode,
Mg 0--> Mg2+ +2e-
Reduction
cathode
Cu+2+20--->Cuo
reduction,
cathode, Zn+2
+2e---> Zno
Reduction
cathode
Ag+1+1o--- Ago
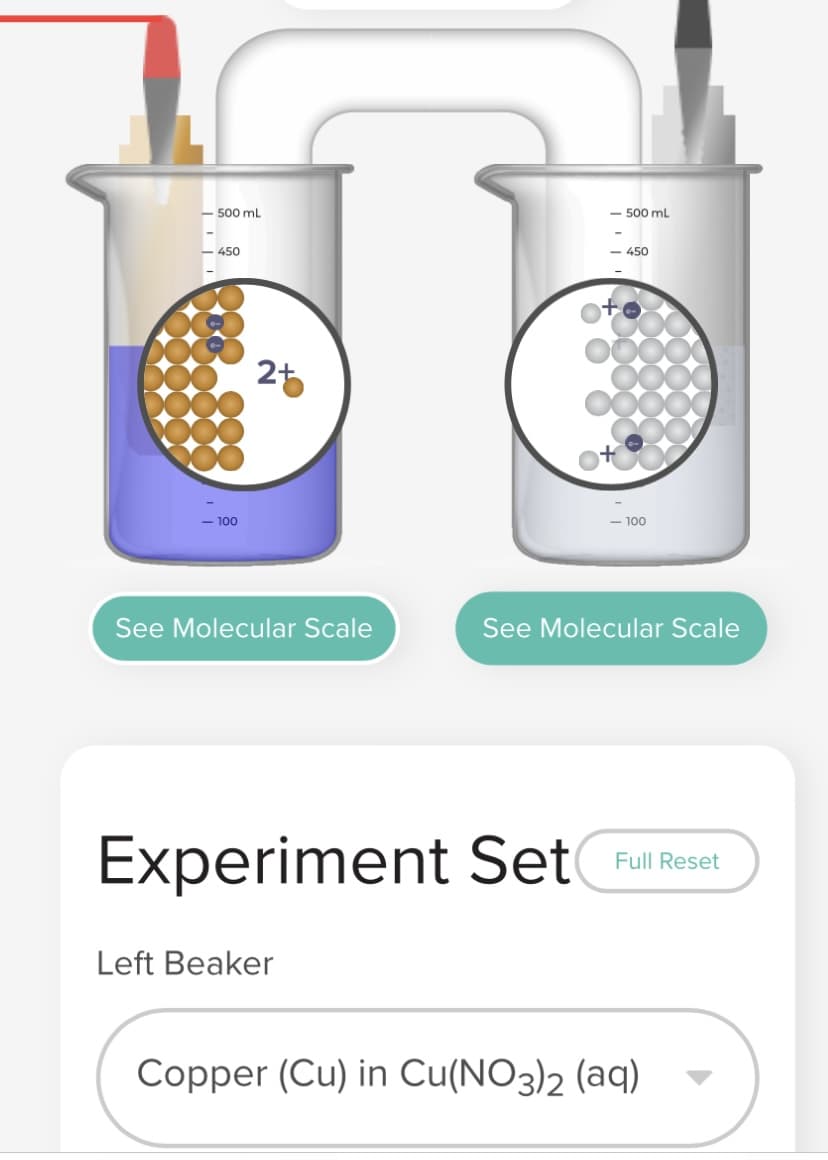
Transcribed Image Text:- 500 mL
450
- 100
25
See Molecular Scale
- 500 mL
Left Beaker
<-450
- 100
See Molecular Scale
Experiment Set Full Reset
Copper (Cu) in Cu(NO3)2 (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

