Write the products of the following acid-base reaction. CH3OCH3 HBr • You should include all products. • If the reaction is a Brønsted acid-base reaction, draw the products in separate sketchers. o Separate multiple products using the + sign from the drop-down menu. • If the reaction is a Lewis acid-base reaction, draw the products in a single sketcher, with a sing • Note: Sodium and potassium never form covalent bonds to electronegative atoms. opy asto CH4 CH4
Write the products of the following acid-base reaction. CH3OCH3 HBr • You should include all products. • If the reaction is a Brønsted acid-base reaction, draw the products in separate sketchers. o Separate multiple products using the + sign from the drop-down menu. • If the reaction is a Lewis acid-base reaction, draw the products in a single sketcher, with a sing • Note: Sodium and potassium never form covalent bonds to electronegative atoms. opy asto CH4 CH4
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.122E
Related questions
Question
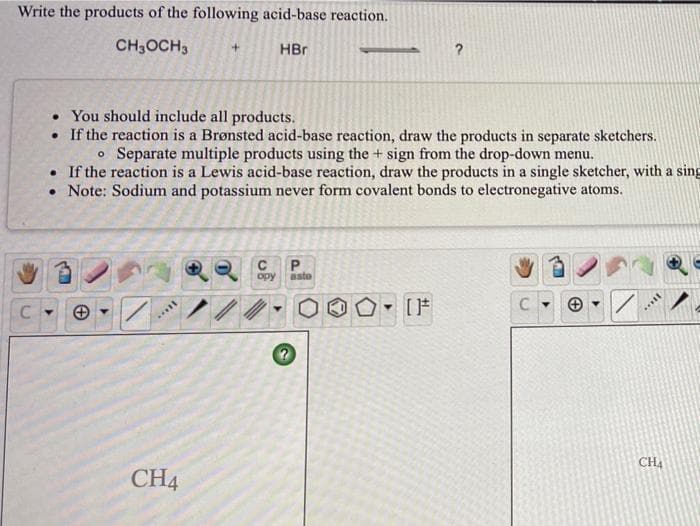
Transcribed Image Text:Write the products of the following acid-base reaction.
CH3OCH3
HBr
• You should include all products.
• If the reaction is a Brønsted acid-base reaction, draw the products in separate sketchers.
o Separate multiple products using the + sign from the drop-down menu.
• If the reaction is a Lewis acid-base reaction, draw the products in a single sketcher, with a sing
• Note: Sodium and potassium never form covalent bonds to electronegative atoms.
opy
aste
CH4
CH4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning