Write the symbol for each of the following particles. (a) an atom with 17 protons and 17 electrons (b) a beryllium atom that has lost 3 electrons (c) a bromine atom that has gained 1 electron (d) a nitrogen atom with 8 neutrons in its nucleus
Write the symbol for each of the following particles. (a) an atom with 17 protons and 17 electrons (b) a beryllium atom that has lost 3 electrons (c) a bromine atom that has gained 1 electron (d) a nitrogen atom with 8 neutrons in its nucleus
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 7A
Related questions
Question
Chapter 2 homework
Transcribed Image Text:A webassign.net
* Lesson.
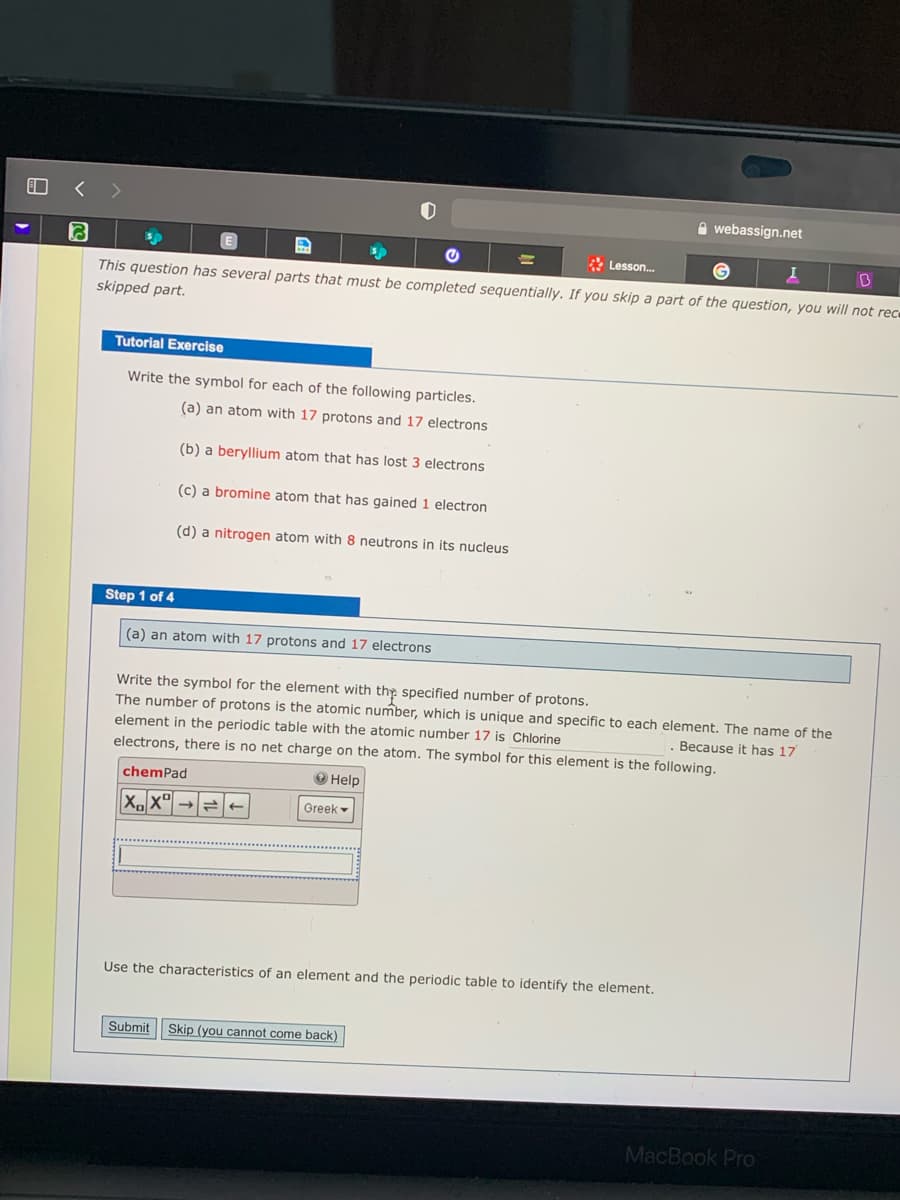
This question has several parts that must be completed sequentially. If you skip a part of the question, you will not rece
skipped part.
Tutorial Exercise
Write the symbol for each of the following particles.
(a) an atom with 17 protons and 17 electrons
(b) a beryllium atom that has lost 3 electrons
(c) a bromine atom that has gained 1 electron
(d) a nitrogen atom with 8 neutrons in its nucleus
Step 1 of 4
(a) an atom with 17 protons and 17 electrons
Write the symbol for the element with the specified number of protons.
The number of protons is the atomic number, which is unique and specific to each element. The name of the
element in the periodic table with the atomic number 17 is Chlorine
electrons, there is no net charge on the atom. The symbol for this element is the following.
Beçause it has 17
chemPad
O Help
Greek -
Use the characteristics of an element and the periodic table to identify the element.
Submit
Skip (you cannot come back)
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning