Writing the net equation for a sequence of reactions OC D 0/5 ARLYC There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC,(s)+2 H,O(g) C,H,(g)+Ca(OH),(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6C,H,(g)+3 CO,(g)-4H,0(g)→5 CH, CHCO,H(g) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced. C-C
Writing the net equation for a sequence of reactions OC D 0/5 ARLYC There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC,(s)+2 H,O(g) C,H,(g)+Ca(OH),(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6C,H,(g)+3 CO,(g)-4H,0(g)→5 CH, CHCO,H(g) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced. C-C
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.77PAE: The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce...
Related questions
Question
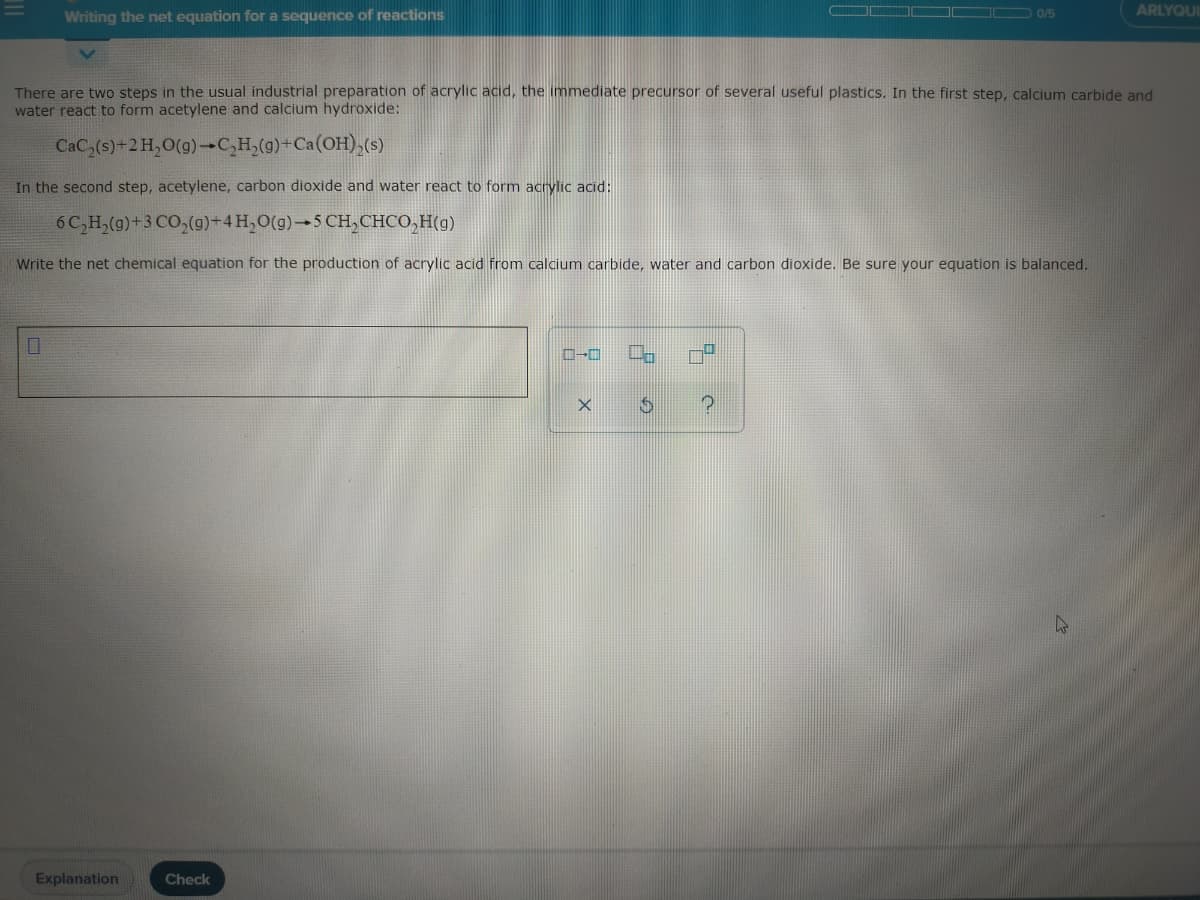
Transcribed Image Text:Writing the net equation for a sequence of reactions
0/5
ARLYQUI
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and
water react to form acetylene and calcium hydroxide:
CaC,(s)+2 H,O(g)-C,H,(g)+Ca(OH),(s)
In the second step, acetylene, carbon dioxide and water react to form acrylic acid:
6 C,H,(g)+3 CO,(g)+4H,O(g)→5 CH,CHCO,H(g)
Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.
C-C
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning