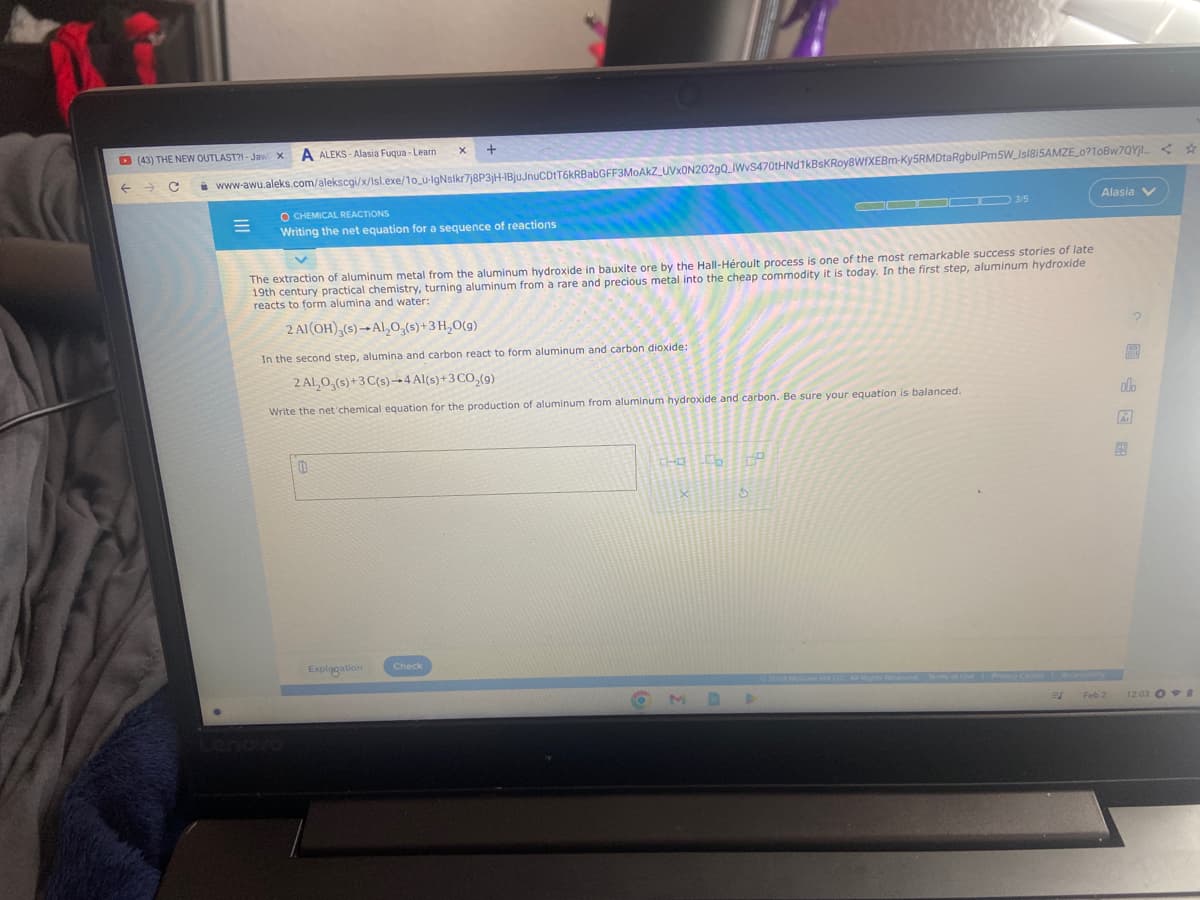
Writing the net equation The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH)3(s)-Al₂O3(s) + 3H₂O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 ALO,(s)+3 C(s)-4 Al(s) + 3 CO₂(g) Write the net 'chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. The Co Op alo
Writing the net equation The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH)3(s)-Al₂O3(s) + 3H₂O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 ALO,(s)+3 C(s)-4 Al(s) + 3 CO₂(g) Write the net 'chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. The Co Op alo
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:A ALEKS-Alasia Fuqua-Learn
=
(43) THE NEW OUTLAST?!-Jaw X
← →
C www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-1BjuJnuCDtT6kRBabGFF3MOAKZ_UVXON202gQ_IWvS470tHNd1kBsKRoy8WfXEBm-Ky5RMDtaRgbulPm5W_Isl815AMZE_0?10Bw7QYJL <
O CHEMICAL REACTIONS
Writing the net equation for a sequence of reactions
X +
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late
19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide
reacts to form alumina and water:
2 Al(OH)3(s)-Al₂O3(s) + 3 H₂O(g)
11
In the second step, alumina and carbon react to form aluminum and carbon dioxide:
2 AL₂O3(s)+3 C(s)-4 Al(s) + 3 CO₂(9)
Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced.
Explagation
Check
THE 60
X
CD 3/5
M
D D
ES
Alasia V
Feb 2
F
allo
12:03 I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

