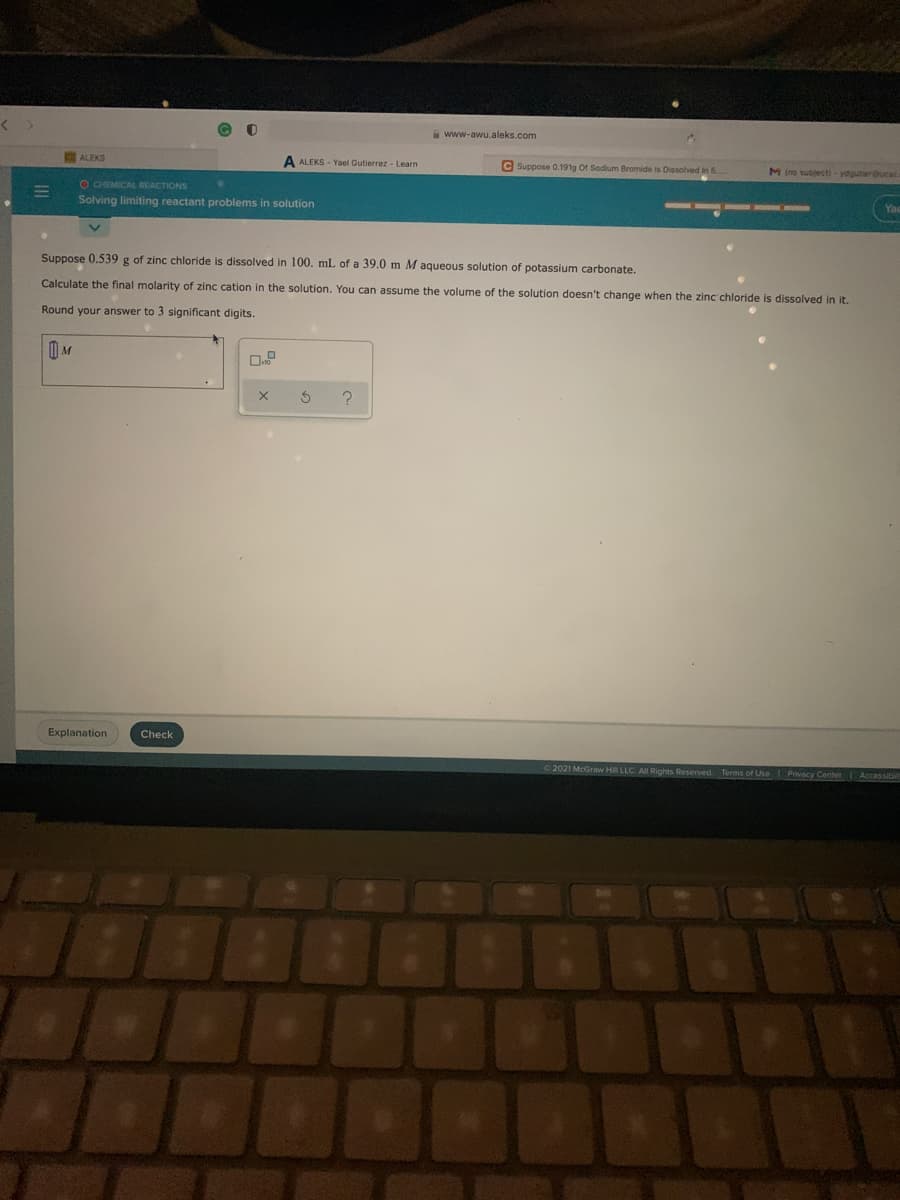
www-awu.aleks.com ALEKS A ALEKS - Yael Gutierrez - Learn C Suppose 0.191g Of Sodium Bromide is Dissolved in 5. M ino sublecti - ydgutier 6OEMICAL REACTIONS Solving limiting reactant problems in solution Suppose 0.539 g of zinc chloride is dissolved in 100. mL of a 39.0 m M aqueous solution of potassium carbonate. Calculate the final molarity of zinc cation in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. Round your answer to 3 significant digits.
www-awu.aleks.com ALEKS A ALEKS - Yael Gutierrez - Learn C Suppose 0.191g Of Sodium Bromide is Dissolved in 5. M ino sublecti - ydgutier 6OEMICAL REACTIONS Solving limiting reactant problems in solution Suppose 0.539 g of zinc chloride is dissolved in 100. mL of a 39.0 m M aqueous solution of potassium carbonate. Calculate the final molarity of zinc cation in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. Round your answer to 3 significant digits.
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
Solving limiting reactant problems in solution.
Transcribed Image Text:<>
a www-awu.aleks.com
ALEKS
A ALEKS - Yael Gutierrez - Learn
C Suppose 0.191g Of Sodium Bromide is Dissolved in 5.
M (no subject) - ydgutier@ucsc
O CHEMICAL REACTIONS
Solving limiting reactant problems in solution
Yac
Suppose 0.539 g of zinc chloride is dissolved in 100. mL of a 39.0 m M aqueous solution of potassium carbonate.
Calculate the final molarity of zinc cation in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it.
Round your answer to 3 significant digits.
| M
Explanation
Check
2021 McGraw HI LLC. AI Rights Reserved Terms of Use Pivacy Center Accessibil
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
