X Home Mail - Esther CeronSotelo - Outlo X (78) How to Calculate Oxidation b Answered: G formula for ammoni X 101 Chem 101 X X app.101edu.co Submit Question 9 of 27 Complete the balanced neutralization equation for the reaction below: NH&OH(aq)HPO.(aq) 3 |2+ 4- |3+ |4+ 1 2 6 7 8 9 0 16 19 4 3 (s) (g) (aq) (1) Н P Reset Delete x H20 4:30 PM е OTYPE here to search 11/14/2019 + LO 2 3 Z
X Home Mail - Esther CeronSotelo - Outlo X (78) How to Calculate Oxidation b Answered: G formula for ammoni X 101 Chem 101 X X app.101edu.co Submit Question 9 of 27 Complete the balanced neutralization equation for the reaction below: NH&OH(aq)HPO.(aq) 3 |2+ 4- |3+ |4+ 1 2 6 7 8 9 0 16 19 4 3 (s) (g) (aq) (1) Н P Reset Delete x H20 4:30 PM е OTYPE here to search 11/14/2019 + LO 2 3 Z
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.20QAP
Related questions
Question
Transcribed Image Text:X
Home
Mail - Esther CeronSotelo - Outlo X
(78) How to Calculate Oxidation
b Answered: G formula for ammoni X
101 Chem 101
X
X
app.101edu.co
Submit
Question 9 of 27
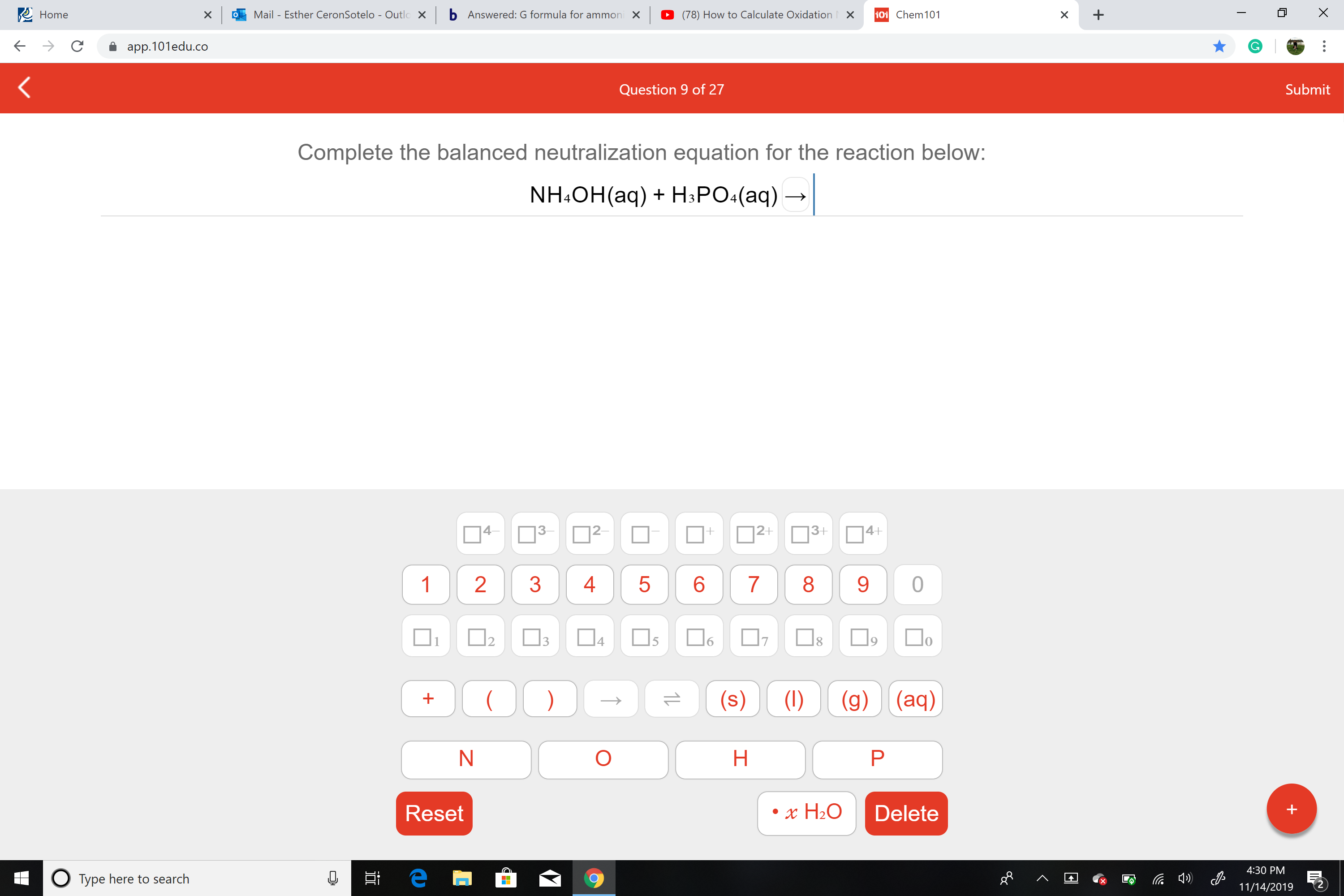
Complete the balanced neutralization equation for the reaction below:
NH&OH(aq)HPO.(aq)
3
|2+
4-
|3+
|4+
1
2
6
7
8
9
0
16
19
4
3
(s)
(g) (aq)
(1)
Н
P
Reset
Delete
x H20
4:30 PM
е
OTYPE here to search
11/14/2019
+
LO
2
3
Z
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning