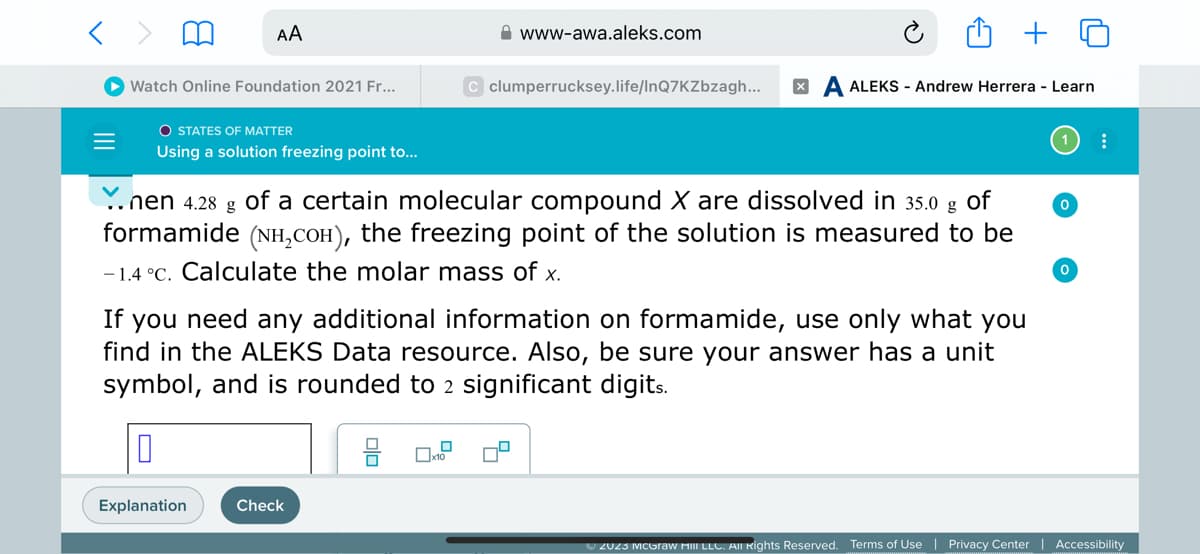
Y. nen 4.28 g of a certain molecular compound X are dissolved in 35.0 g of formamide (NH₂COH), the freezing point of the solution is measured to be -1.4 °C. Calculate the molar mass of x. If you need any additional information on formamide, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 0 8 0..9 0 0
Y. nen 4.28 g of a certain molecular compound X are dissolved in 35.0 g of formamide (NH₂COH), the freezing point of the solution is measured to be -1.4 °C. Calculate the molar mass of x. If you need any additional information on formamide, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 0 8 0..9 0 0
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 66QAP: The Henry's law constant for the solubility of radon in water at is 9.57106 M/mm Hg. Radon is...
Related questions
Question
Freezing point for molar mass.
Do not round mid equation because Aleks flags it. Round at the end to appropriate sig figs
Transcribed Image Text:< >
=
AA
Watch Online Foundation 2021 Fr...
O STATES OF MATTER
Using a solution freezing point to...
Explanation
nen 4.28 g of a certain molecular compound X are dissolved in 35.0 g
of
formamide (NH₂COн), the freezing point of the solution is measured to be
-1.4 °C. Calculate the molar mass of x.
www-awa.aleks.com
If you need any additional information on formamide, use only what you
find in the ALEKS Data resource. Also, be sure your answer has a unit
symbol, and is rounded to 2 significant digits.
0
Check
C clumperrucksey.life/InQ7KZbzagh... XA ALEKS- Andrew Herrera - Learn
☐x10
0
0
:
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,