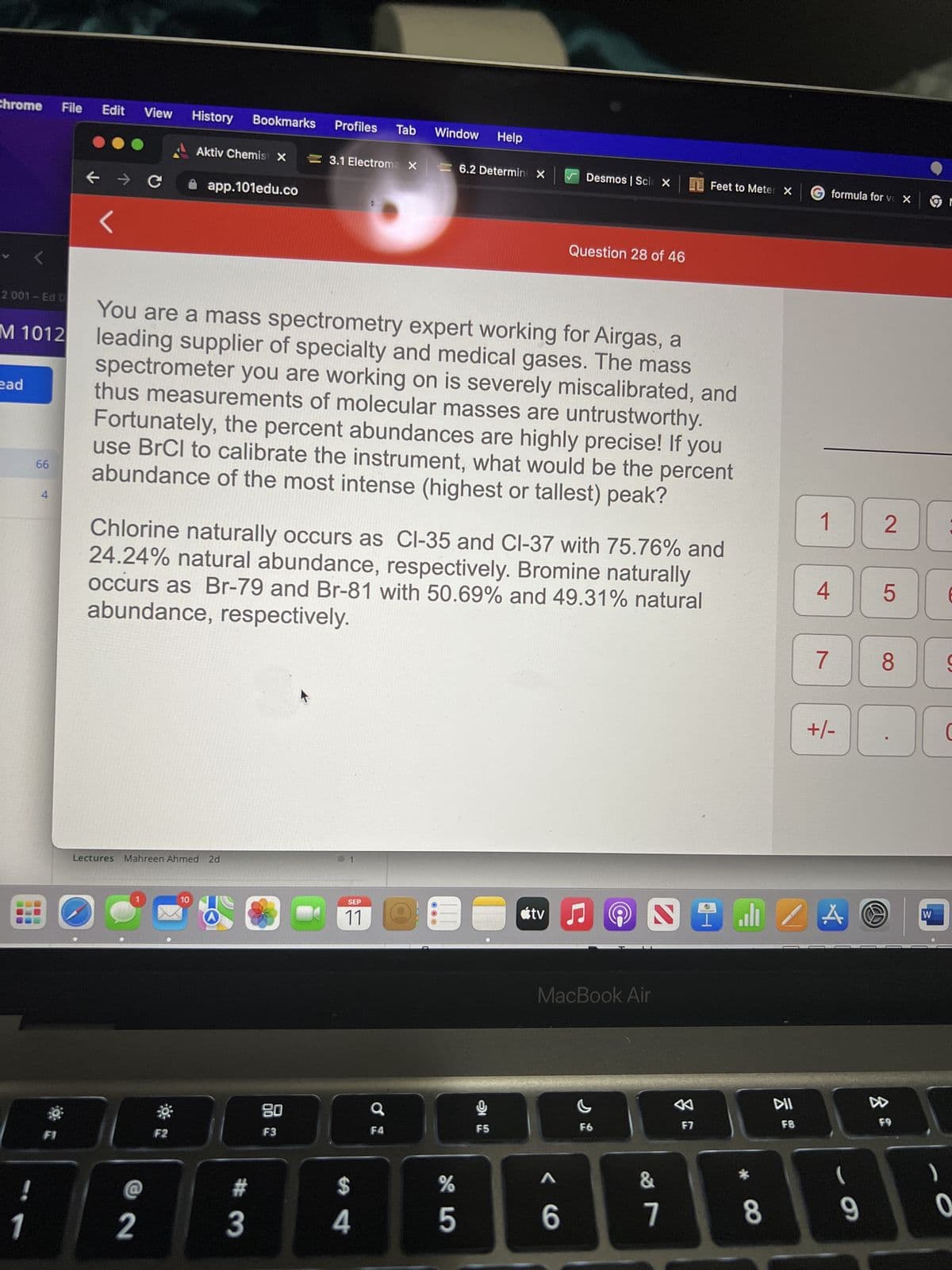
You are a mass spectrometry expert working for Airgas, a leading supplier of specialty and medical gases. The mass spectrometer you are working on is severely miscalibrated, and thus measurements of molecular masses are untrustworthy. Fortunately, the percent abundances are highly precise! If you use BrCl to calibrate the instrument, what would be the percent abundance of the most intense (highest or tallest) peak? Chlorine naturally occurs as Cl-35 and Cl-37 with 75.76% and 24.24% natural abundance, respectively. Bromine naturally occurs as Br-79 and Br-81 with 50.69% and 49.31% natural abundance, respectively.
You are a mass spectrometry expert working for Airgas, a leading supplier of specialty and medical gases. The mass spectrometer you are working on is severely miscalibrated, and thus measurements of molecular masses are untrustworthy. Fortunately, the percent abundances are highly precise! If you use BrCl to calibrate the instrument, what would be the percent abundance of the most intense (highest or tallest) peak? Chlorine naturally occurs as Cl-35 and Cl-37 with 75.76% and 24.24% natural abundance, respectively. Bromine naturally occurs as Br-79 and Br-81 with 50.69% and 49.31% natural abundance, respectively.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 90QAP: Calculate the average density of a single Al-27 atom by assuming that it is a sphere with a radius...
Related questions
Question
Transcribed Image Text:Chrome File
2 001- Ed D
M 1012
ead
!
1
66
4
F1
Edit View
← → C
History
2
F2
Aktiv Chemist X
Lectures Mahreen Ahmed 2d
10
Bookmarks
app.101edu.co
#3
Profiles Tab
3.1 Electroma X
You are a mass spectrometry expert working for Airgas, a
leading supplier of specialty and medical gases. The mass
spectrometer you are working on is severely miscalibrated, and
thus measurements of molecular masses are untrustworthy.
Fortunately, the percent abundances are highly precise! If you
use BrCl to calibrate the instrument, what would be the percent
abundance of the most intense (highest or tallest) peak?
80
F3
Chlorine naturally occurs as Cl-35 and Cl-37 with 75.76% and
24.24% natural abundance, respectively. Bromine naturally
occurs as Br-79 and Br-81 with 50.69% and 49.31% natural
abundance, respectively.
SEP
11
$
4
Window Help
F4
Q
6.2 Determini X
%
45
2
F5
Desmos | Sci X
Question 28 of 46
tv ♫
A
6
MacBook Air
F6
2
&
Feet to Meter X G formula for v X
7
A
NiZA
F7
(
*
8
1
DII
F8
4
7
+/-
61
2
5
8
F9
W
1
F
6
S
C
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning