You are assigned the task of separating a desired granular material, with a density of 3.26 g/cm°, from an undesired granular material that has a density of 2.04 g/cm3. You want to do this by shaking the mixture in a liquid. A solid will float on any liquid that is more dense. Which of these liquids will serve your purpose, assuming no chemical interaction between the liquid and the solids? O Carbon tetrachloride (1.59 g/ml) O Hexane (0.66 g/ml) O Bromoform (2.89 g/ml) O Diiodomethane (3.32 g/ml) O Benzene (0.88 g/ml)
You are assigned the task of separating a desired granular material, with a density of 3.26 g/cm°, from an undesired granular material that has a density of 2.04 g/cm3. You want to do this by shaking the mixture in a liquid. A solid will float on any liquid that is more dense. Which of these liquids will serve your purpose, assuming no chemical interaction between the liquid and the solids? O Carbon tetrachloride (1.59 g/ml) O Hexane (0.66 g/ml) O Bromoform (2.89 g/ml) O Diiodomethane (3.32 g/ml) O Benzene (0.88 g/ml)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 52GQ
Related questions
Question
Picture has the question
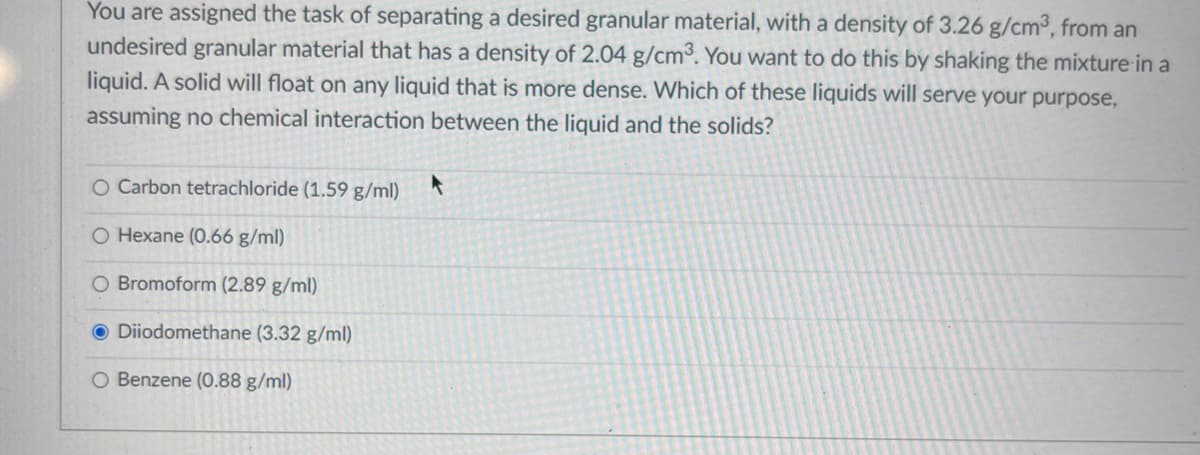
Transcribed Image Text:You are assigned the task of separating a desired granular material, with a density of 3.26 g/cm³, from an
undesired granular material that has a density of 2.04 g/cm³. You want to do this by shaking the mixture in a
liquid. A solid will float on any liquid that is more dense. Which of these liquids will serve your purpose,
assuming no chemical interaction between the liquid and the solids?
O Carbon tetrachloride (1.59 g/ml)
O Hexane (0.66 g/ml)
O Bromoform (2.89 g/ml)
O Diiodomethane (3.32 g/ml)
O Benzene (0.88 g/ml)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning