You have a 1.153g sample of an unknown solid acid, HA, dissolved in enough water to make 20.00 mLof solution. HA reacts with KOH(ad) according to the following balanced chemical equation: HA(aq) + KOH(aq) → KA(aq) +H,0(1) 1st attempt Part 1(. H See Periodic Table O See Hint If 13.15 mL of 0.655 M KOH is required to titrate the unknown acid to the equivalence point, what is the concentration of the unknown acid? Part 2 (* O See Hint What is the molar mass of HA? g/mol MN
You have a 1.153g sample of an unknown solid acid, HA, dissolved in enough water to make 20.00 mLof solution. HA reacts with KOH(ad) according to the following balanced chemical equation: HA(aq) + KOH(aq) → KA(aq) +H,0(1) 1st attempt Part 1(. H See Periodic Table O See Hint If 13.15 mL of 0.655 M KOH is required to titrate the unknown acid to the equivalence point, what is the concentration of the unknown acid? Part 2 (* O See Hint What is the molar mass of HA? g/mol MN
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 121AP: Calcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion...
Related questions
Question
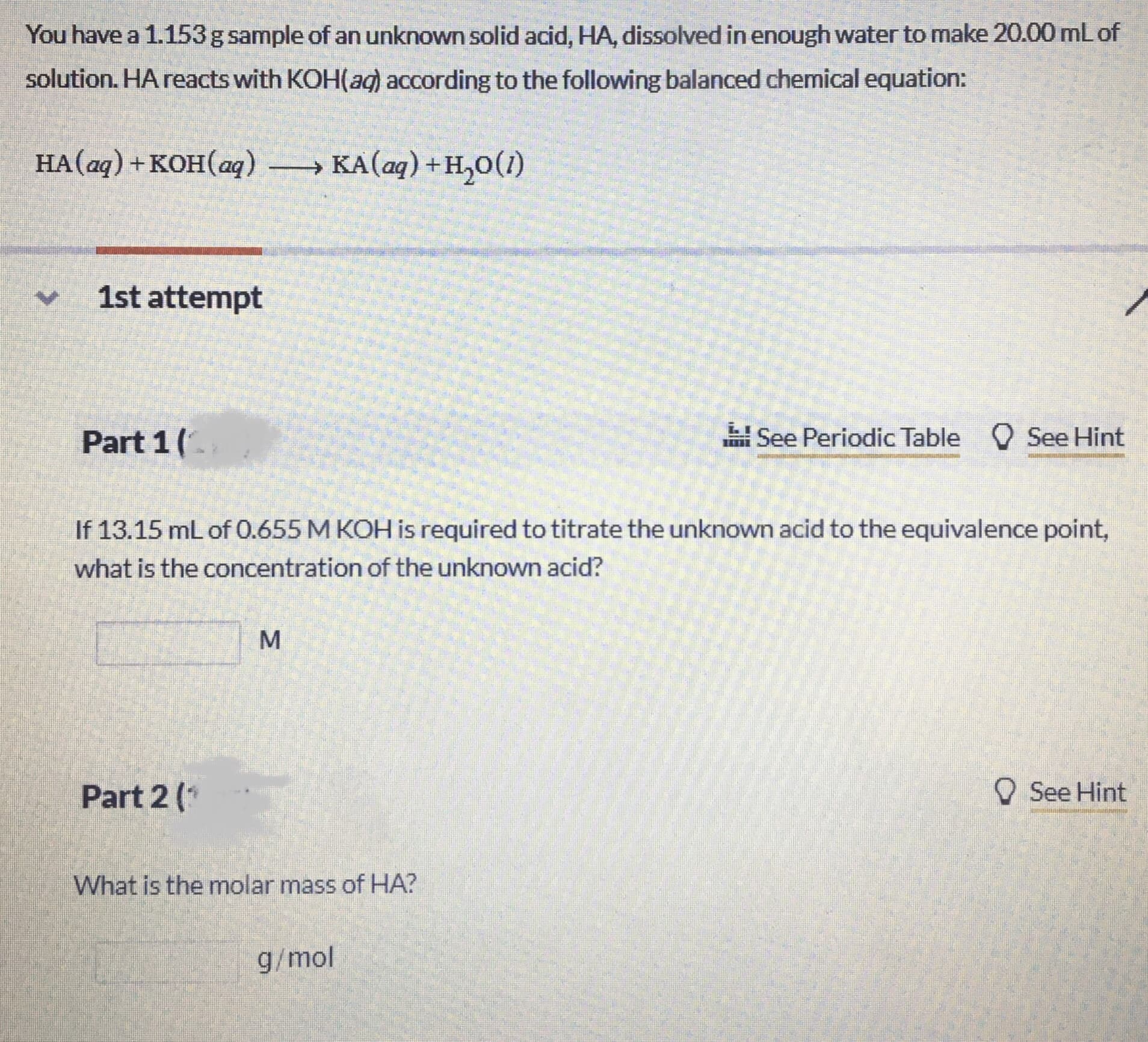
Transcribed Image Text:You have a 1.153g sample of an unknown solid acid, HA, dissolved in enough water to make 20.00 mLof
solution. HA reacts with KOH(ad) according to the following balanced chemical equation:
HA(aq) + KOH(aq) → KA(aq) +H,0(1)
1st attempt
Part 1(.
H See Periodic Table O See Hint
If 13.15 mL of 0.655 M KOH is required to titrate the unknown acid to the equivalence point,
what is the concentration of the unknown acid?
Part 2 (*
O See Hint
What is the molar mass of HA?
g/mol
MN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning