Request Request 6.5 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate solution, the initial volume on the burette is 0.82 ml and the final volume is 18.15 ml. What is the number of moles of borate in the solution? Hint the reaction is equation # 4 on page 3 of the laboratory manual for this experiment. (This data will be repeated in the next question.) (Give 4 digits of the calculator answer) O a. 0.003366 mol Ob. 0.08426 mol O c. none of these O d. 6.733 mol O e. 17.33 mol O f. 0.006733 mol O g. 9.730 mol Clear my choice 9 uo et 6.6 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate solution, the initial volume on the burette is 0.82 ml and the final volume is 18.15 ml. What is the molarity of this borate solution? (This data will be used in is out of the next question.) (Give 4 digits of the calculator answer) Flag O a. 0.01258 M estion Ob. 0.5024 M O c. 0.08426 M d. none of these 1.452 M e. f. 1.005 M 0.001005 M g. MacBook Air
Request Request 6.5 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate solution, the initial volume on the burette is 0.82 ml and the final volume is 18.15 ml. What is the number of moles of borate in the solution? Hint the reaction is equation # 4 on page 3 of the laboratory manual for this experiment. (This data will be repeated in the next question.) (Give 4 digits of the calculator answer) O a. 0.003366 mol Ob. 0.08426 mol O c. none of these O d. 6.733 mol O e. 17.33 mol O f. 0.006733 mol O g. 9.730 mol Clear my choice 9 uo et 6.6 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate solution, the initial volume on the burette is 0.82 ml and the final volume is 18.15 ml. What is the molarity of this borate solution? (This data will be used in is out of the next question.) (Give 4 digits of the calculator answer) Flag O a. 0.01258 M estion Ob. 0.5024 M O c. 0.08426 M d. none of these 1.452 M e. f. 1.005 M 0.001005 M g. MacBook Air
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 6ALQ: onsider separate aqueous solutions of HCI and H2S04 with the same concentrations in terms of...
Related questions
Question
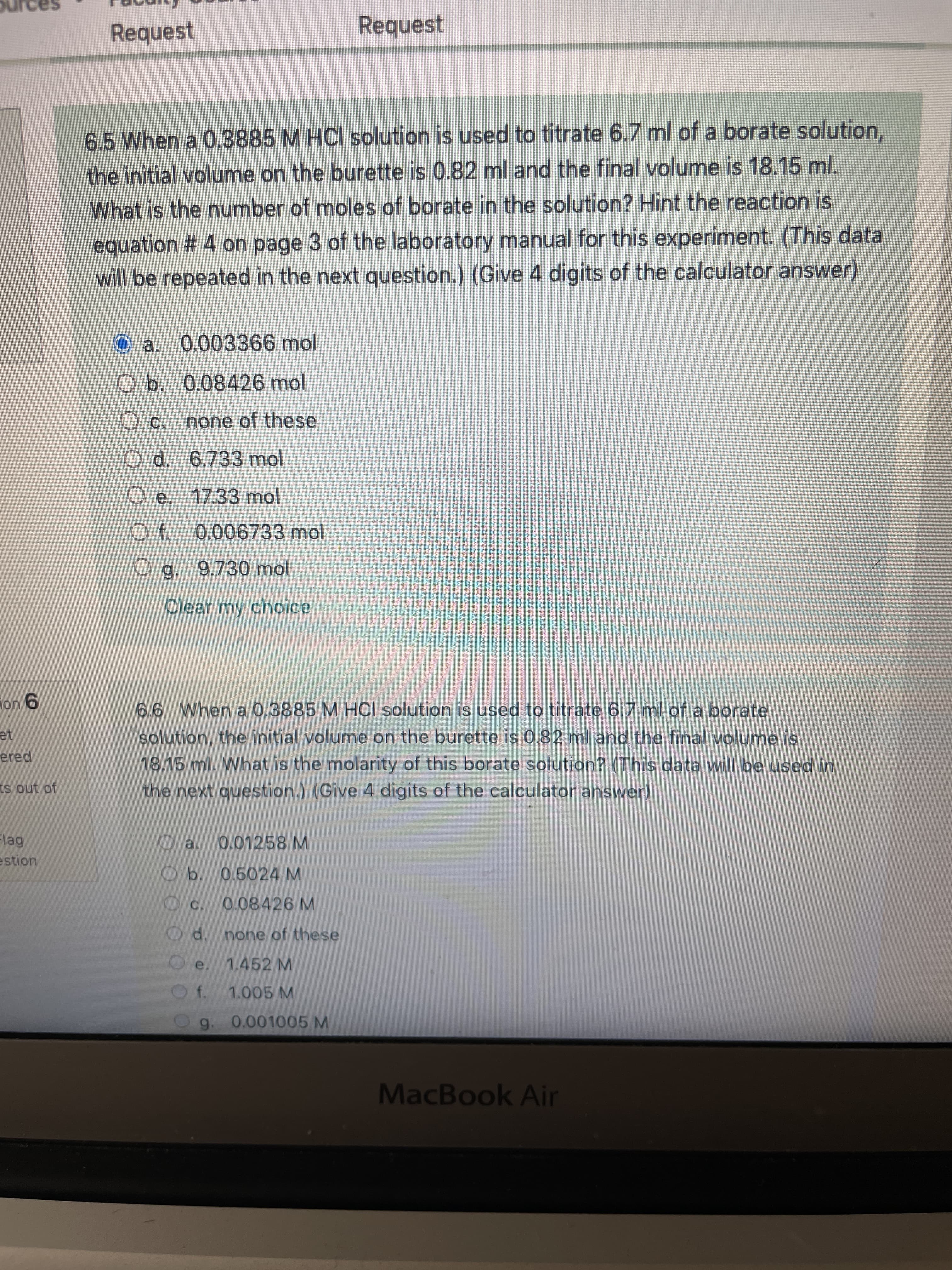
Transcribed Image Text:Request
Request
6.5 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate solution,
the initial volume on the burette is 0.82 ml and the final volume is 18.15 ml.
What is the number of moles of borate in the solution? Hint the reaction is
equation # 4 on page 3 of the laboratory manual for this experiment. (This data
will be repeated in the next question.) (Give 4 digits of the calculator answer)
O a. 0.003366 mol
Ob. 0.08426 mol
O c. none of these
O d. 6.733 mol
O e. 17.33 mol
O f. 0.006733 mol
O g. 9.730 mol
Clear my choice
9 uo
et
6.6 When a 0.3885 M HCI solution is used to titrate 6.7 ml of a borate
solution, the initial volume on the burette is 0.82 ml and the final volume is
18.15 ml. What is the molarity of this borate solution? (This data will be used in
is out of
the next question.) (Give 4 digits of the calculator answer)
Flag
O a. 0.01258 M
estion
Ob. 0.5024 M
O c. 0.08426 M
d.
none of these
1.452 M
e.
f. 1.005 M
0.001005 M
g.
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
