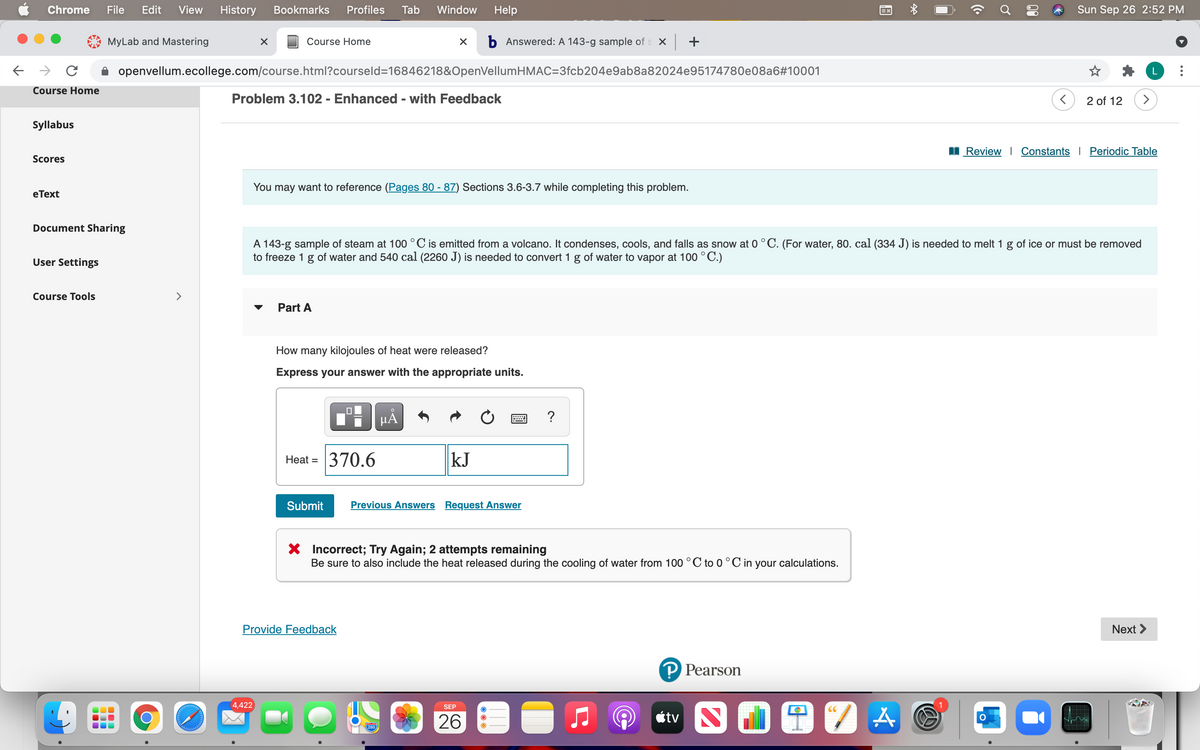
You may want to reference (Pages 80 - 87) Sections 3.6-3.7 while completing this problem. A 143-g sample of steam at 100 °C is emitted from a volcano. It condenses, cools, and falls as snow at 0 °C. (For water, 80. cal (334 J) is needed to melt 1 g of ice or must be removed to freeze 1 g of water and 540 cal (2260 J) is needed to convert 1 g of water to vapor at 100 °C.) Part A How many kilojoules of heat were released? Express your answer with the appropriate units. HẢ Heat = 370.6 kJ Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remaining
You may want to reference (Pages 80 - 87) Sections 3.6-3.7 while completing this problem. A 143-g sample of steam at 100 °C is emitted from a volcano. It condenses, cools, and falls as snow at 0 °C. (For water, 80. cal (334 J) is needed to melt 1 g of ice or must be removed to freeze 1 g of water and 540 cal (2260 J) is needed to convert 1 g of water to vapor at 100 °C.) Part A How many kilojoules of heat were released? Express your answer with the appropriate units. HẢ Heat = 370.6 kJ Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remaining
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section15.6: Disturbing A Chemical Equilibrium
Problem 1.1ACP: Anhydrous ammonia is used directly as a fertilizer, but much of it is also converted to other...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
Sun Sep 26 2:52 PM
MyLab and Mastering
Course Home
b Answered: A 143-g sample of s x +
openvellum.ecollege.com/course.html?courseld=16846218&OpenVellumHMAC=3fcb204e9ab8a82024e95174780e08a6#10001
L
Course Home
Problem 3.102 - Enhanced - with Feedback
2 of 12
>
Syllabus
I Review | Constants | Periodic Table
Scores
You may want to reference (Pages 80 - 87) Sections 3.6-3.7 while completing this problem.
eТext
Document Sharing
A 143-g sample of steam at 100 °C is emitted from a volcano. It condenses, cools, and falls as snow at 0°C. (For water, 80. cal (334 J) is needed to melt 1 g of ice or must be removed
to freeze 1 g of water and 540 cal (2260 J) is needed to convert 1 g of water to vapor at 100 °C.)
User Settings
Course Tools
>
Part A
How many kilojoules of heat were released?
Express your answer with the appropriate units.
?
Heat = 370.6
kJ
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 2 attempts remaining
Be sure to also include the heat released during the cooling of water from 100 °C to 0°C in your calculations.
Provide Feedback
Next >
P Pearson
4,422
SEP
1
CC
ktv
26
280
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning