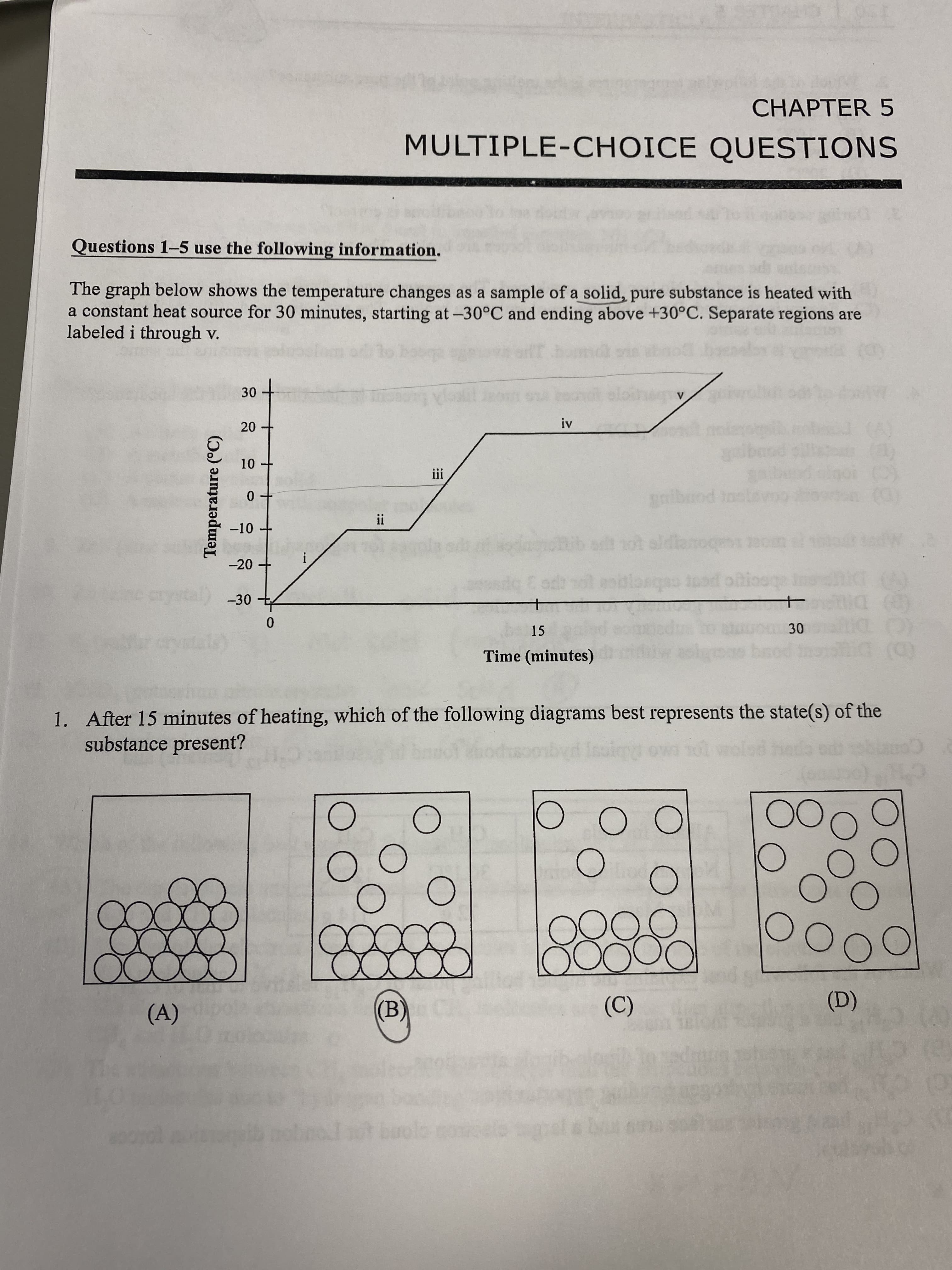
10 Temperature (°C) CHAPTER 5 MULTIPLE-CHOICE QUESTIONS Questions 1-5 use the following information. The graph below shows the temperature changes as a sample of a solid, pure substance is heated with a constant heat source for 30 minutes, starting at -30°C and ending above +30°C. Separate regions are labeled i through v. iii gnibrod in -10 I! t ot aldianoqe1 200m -20 crystal) -30 15 Time (minutes) (D) 1. After 15 minutes of heating, which of the following diagrams best represents the state(s) of the substance present? chec C'H 00 00 (A) (D) (B) (C) buolo comele 7. Unauthorized copying or reusing any part of this page is Illegal. CHAPTER 5 120 2. Which of the following temperatures is the melting point of the pure substance? (A) -30°C (B) -10°C ITJUM CHO or (D) 20°C (A) No energy is absorbed. No interparticle forces are broken. The average speed of the molecules remains the same. 3. During section ii of the heating curve, which set of conditions is correct? (B) Energy is absorbed. No bonds are weakened. The average speed of the molecules increases. (C) Energy is absorbed. Bonds between molecules are broken. The average speed of the molecules remains the same. (D) Energy is released. Bonds are formed. The average speed of the molecules remains the same. 4. Which of the following interparticle forces are.most likely present in the solid form of the substance? (A) London dispersion forces (LDF) (B) metallic bonding (C) ionic bonding (D) network covalent bonding 5. What factor is most responsible for the differences in the slopes for regions i, iii and v? (A) Different specific heat capacities for the 3 phases. (B) Different molecular geometry for the molecules. (C) Different amounts of substance being heated. (D) Different bond energies within the molecules. 6. Consider the chart below for two typical hydrocarbons found in gasoline: C,H,, (pentane) and C3H18 (octane). 12 Alkane formula Normal boiling point 12 C3H18 36.1°C 127°C Molar Mass 114 g Which of the following best explains the higher boiling point of C,H,3 relative to that of C,H2? (A) C,H13 has a greater molar mass. (B) C3H18 has a greater number of dipole-dipole attractions. (C) C,H13 has more hydrogen bonding opportunities. (D) CH13 has a greater surface area and a larger electron cloud for London dispersion forces to develop. 18
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









