Your laboratory instructor has given you two metal cylinders. The mass of each sample was determined on a balance to five significant figures. The volume was determined by (a) measuring the dimensions of the cylinders and (b) immersing the samples in a graduated cylinder as shown in the figure below. The initial volume of water was 25.0 mL. The uncertainty of the volume measurement using the graduated cylinder is +/-0.1 mL. Cylinder A Cylinder B 40 40 30 30 20 20 10 10 Mass (g) Height (cm) Diameter (cm) Cylinder A 15.560 5.11 1.20 Cylinder B 35.536 5.90 1.30 Which statements are true about the number of significant figures in the volume measurements? The dimension method volume has one significant figure. The dimension method volume has two significant figures. The dimension method volume has three significant figures. The water displacement volume has one significant figure. The water displacement volume has two significant figures. The water displacement volume has three significant figures. 000000
Your laboratory instructor has given you two metal cylinders. The mass of each sample was determined on a balance to five significant figures. The volume was determined by (a) measuring the dimensions of the cylinders and (b) immersing the samples in a graduated cylinder as shown in the figure below. The initial volume of water was 25.0 mL. The uncertainty of the volume measurement using the graduated cylinder is +/-0.1 mL. Cylinder A Cylinder B 40 40 30 30 20 20 10 10 Mass (g) Height (cm) Diameter (cm) Cylinder A 15.560 5.11 1.20 Cylinder B 35.536 5.90 1.30 Which statements are true about the number of significant figures in the volume measurements? The dimension method volume has one significant figure. The dimension method volume has two significant figures. The dimension method volume has three significant figures. The water displacement volume has one significant figure. The water displacement volume has two significant figures. The water displacement volume has three significant figures. 000000
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 21RPS: You and your lab partner are asked to determine the density of an aluminum bar. The mass is known...
Related questions
Question
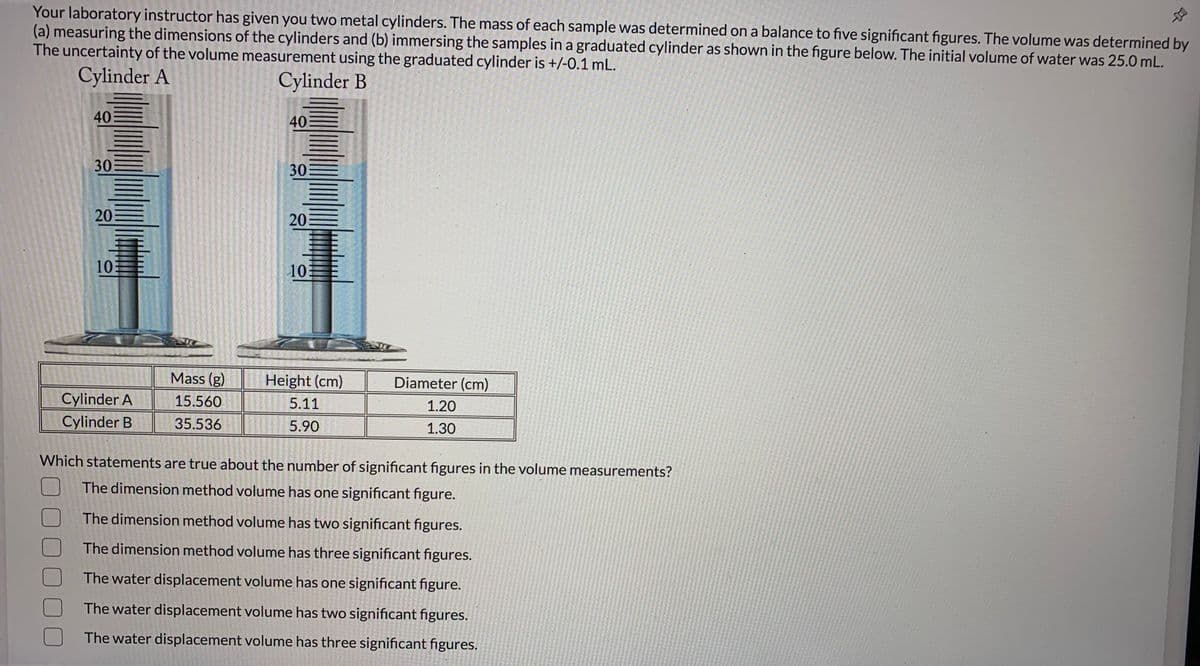
Transcribed Image Text:Your laboratory instructor has given you two metal cylinders. The mass of each sample was determined on a balance to five significant figures. The volume was determined by
(a) measuring the dimensions of the cylinders and (b) immersing the samples in a graduated cylinder as shown in the figure below. The initial volume of water was 25.0 mL.
The uncertainty of the volume measurement using the graduated cylinder is +/-0.1 mL.
Cylinder A
Cylinder B
40
40
30
30
20
20
10
10
Mass (g)
Height (cm)
Diameter (cm)
Cylinder A
15.560
5.11
1.20
Cylinder B
35.536
5.90
1.30
Which statements are true about the number of significant figures in the volume measurements?
The dimension method volume has one significant figure.
The dimension method volume has two significant figures.
The dimension method volume has three significant figures.
The water displacement volume has one significant figure.
The water displacement volume has two significant figures.
The water displacement volume has three significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning