YouTube Remaining Time: 1 hour, 06 minutes, 40 seconds. * Question Completion Status: ВFЗ QUESTION 13 What volume of 0.0347 M Ba(OH)2 is needed to completely react with 71.5 mL of 0.0435 M HCI? 2 HCI + Ba(OH)2 → BaCl2 + 2 H2O 89.6 mL O 179 mL O 28.5 mL O 44.8 mL QUESTION 14 What is the molarity of a Ca(OH)2 solution if 27.5 mL of the solution are required to completely rea of 0.148 M Н3РОД? Click Save and Submit to save and submit. Click Save All Answers to save all answers. oll to see more 2,466 APR 15 3D
YouTube Remaining Time: 1 hour, 06 minutes, 40 seconds. * Question Completion Status: ВFЗ QUESTION 13 What volume of 0.0347 M Ba(OH)2 is needed to completely react with 71.5 mL of 0.0435 M HCI? 2 HCI + Ba(OH)2 → BaCl2 + 2 H2O 89.6 mL O 179 mL O 28.5 mL O 44.8 mL QUESTION 14 What is the molarity of a Ca(OH)2 solution if 27.5 mL of the solution are required to completely rea of 0.148 M Н3РОД? Click Save and Submit to save and submit. Click Save All Answers to save all answers. oll to see more 2,466 APR 15 3D
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 125QRT: A student set up an experiment for six different trials of the reaction between 1.00-M aqueous...
Related questions
Question
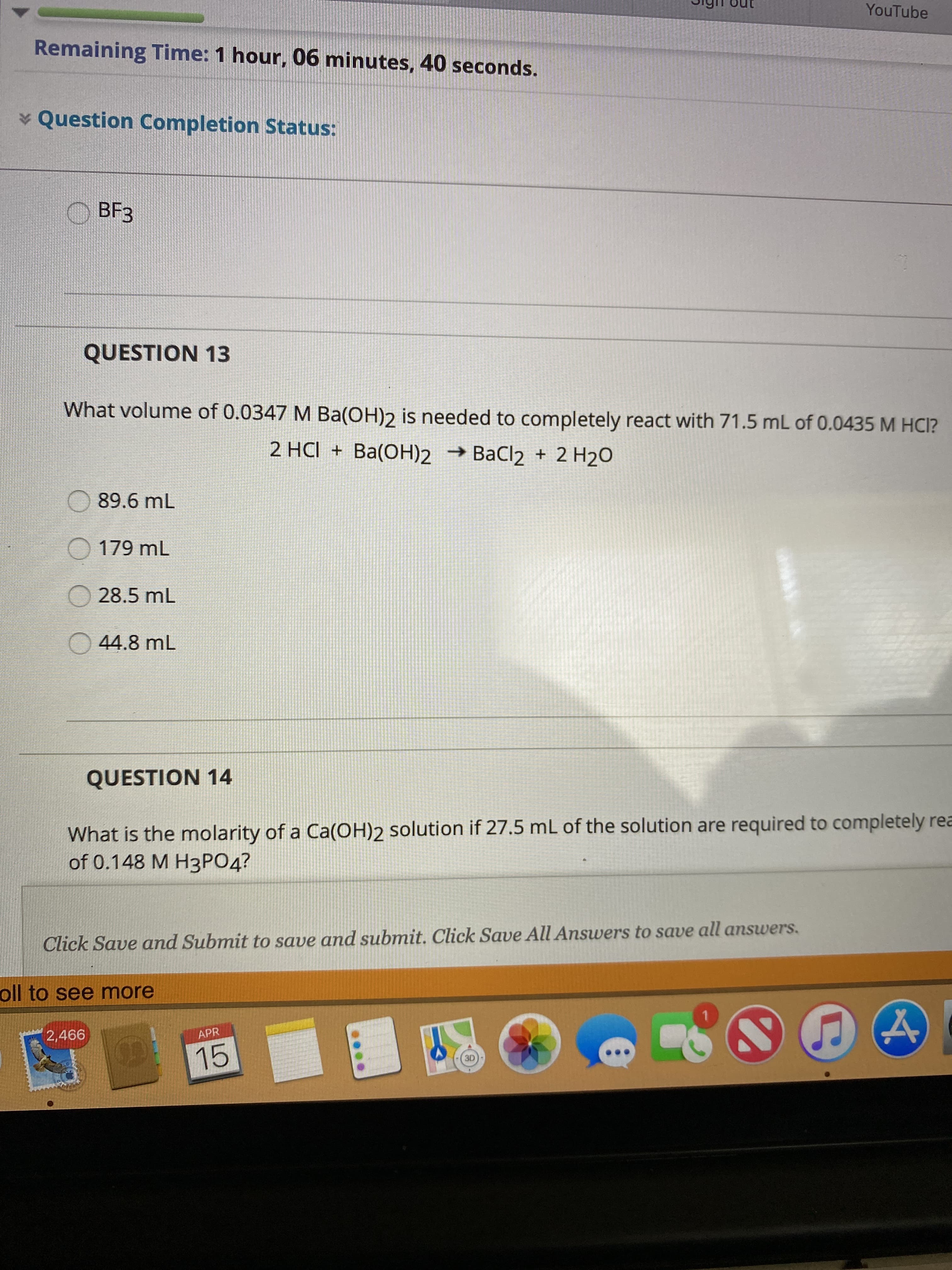
Transcribed Image Text:YouTube
Remaining Time: 1 hour, 06 minutes, 40 seconds.
* Question Completion Status:
ВFЗ
QUESTION 13
What volume of 0.0347 M Ba(OH)2 is needed to completely react with 71.5 mL of 0.0435 M HCI?
2 HCI + Ba(OH)2 → BaCl2 + 2 H2O
89.6 mL
O 179 mL
O 28.5 mL
O 44.8 mL
QUESTION 14
What is the molarity of a Ca(OH)2 solution if 27.5 mL of the solution are required to completely rea
of 0.148 M Н3РОД?
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
oll to see more
2,466
APR
15
3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
