ype of chemical reaction that involves a transter of electrons between wo species * oxidation-reduction polytropic complex D chemical equilibrium Vhich of the following statement is TRUE? * All voltaic (galvanic) cells involve the use of electricity to initiate nonspontaneous chemical reactions. OOxidation and reduction half-reactions occur at electrodes in electrochemical cel
ype of chemical reaction that involves a transter of electrons between wo species * oxidation-reduction polytropic complex D chemical equilibrium Vhich of the following statement is TRUE? * All voltaic (galvanic) cells involve the use of electricity to initiate nonspontaneous chemical reactions. OOxidation and reduction half-reactions occur at electrodes in electrochemical cel
Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.3QAP
Related questions
Question
100%
please correct me if my answers are wrong pleaseeeee..
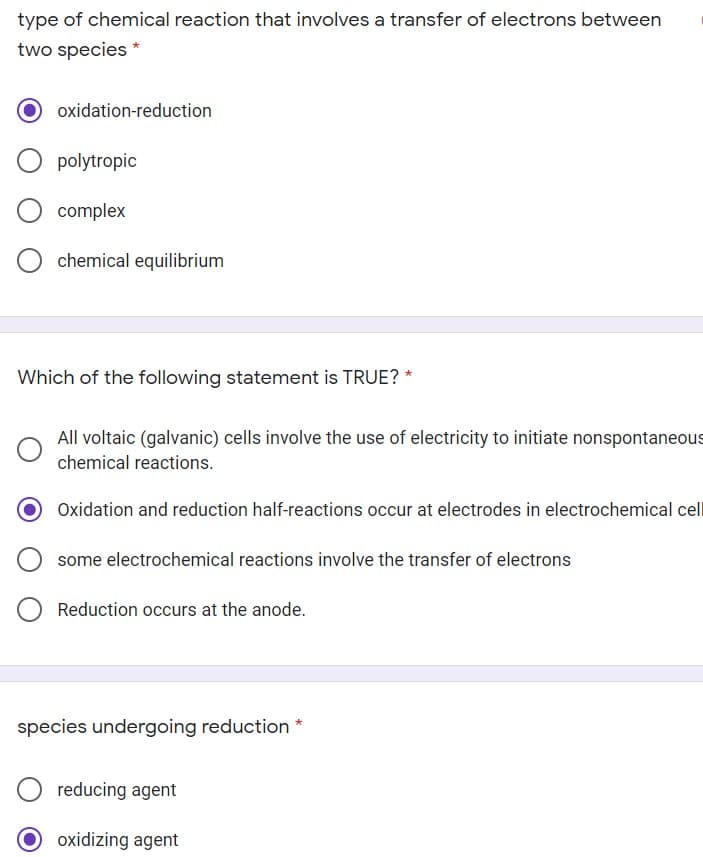
Transcribed Image Text:type of chemical reaction that involves a transfer of electrons between
two species *
oxidation-reduction
polytropic
complex
chemical equilibrium
Which of the following statement is TRUE? *
All voltaic (galvanic) cells involve the use of electricity to initiate nonspontaneous
chemical reactions.
Oxidation and reduction half-reactions occur at electrodes in electrochemical cel
some electrochemical reactions involve the transfer of electrons
Reduction occurs at the anode.
species undergoing reduction
reducing agent
O oxidizing agent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax