38. The following graph shows the historical concentration of atmospheric carbon dioxide in the atmosphere. Carbon dioxide concentrations began increasing in the 1800s as humans started to rely on combustion for energy (carbon dioxide is a product of combustion). The increase in atmospheric carbon dioxide is linked to the increases in global temperatures known as global warming 380 360 340 320 300 280 260 1700 1800 1900 2000 Year Atmospheric carbon dioxide derived from Siple Station ice core measurements and from levels measured at the South Pole, Antarctica (Source: ORNL/CDIAC65) a. What is the total increase in carbon dioxide between 1950 and 2007? b. What is the average yearly increase in carbon dioxide over this period? c. What is the total percentage increase in carbon dioxide over this period? d. What is the average yearly percentage increase in carbon dioxide over this period? CO2 concentration (ppm)
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
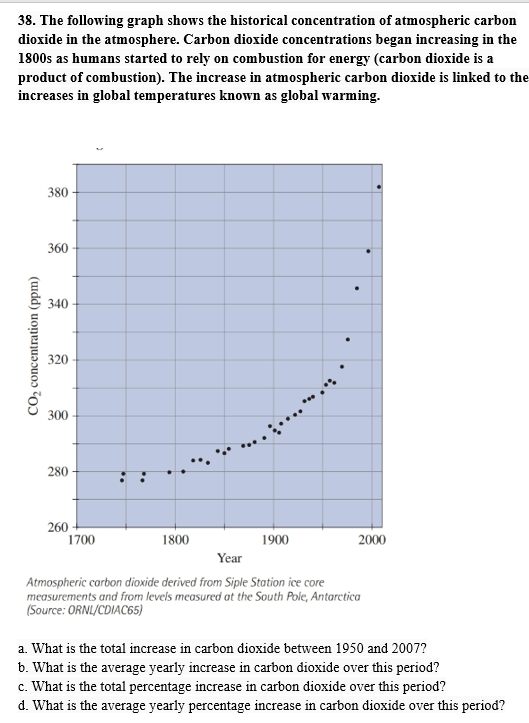
The following graph shows the historical concentration of atmospheric carbon dioxide in the atmosphere. Carbon dioxide concentrations began increasing in the 1800s as humans started to rely on combustion for energy (carbon dioxide is a product of combustion). The increase in atmospheric carbon dioxide is linked to the increases in global temperatures known as global warming.
- What is the total increase in carbon dioxide between 1950 and 2007?
- What is the average yearly increase in carbon dioxide over this period?
- What is the total percentage increase in carbon dioxide over this period?
- What is the average yearly percentage increase in carbon dioxide over this period?
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images









