ons/17136 V/suonsənb/əxeı/6 177 1. This is the data sheet for Molar mass of vapor; 1. Unknown Liquid #:N/A 2. Mass of flask+ cap + rubber band 56.45 g se 3. Temperature of boiling water 97 °C ३ 4. Barometric Pressure 755.7 mmHg 5. Mass of flask + rubber band + cap + condensed vapor_57.88 6. Mass of condensed vapor 7. Volume of flask 243.8 mL 8. Molar Mass of Vapor g/mol What is the molar mass of vapor? O 179200 O 1.79 0179.2 N beatsqudio
ons/17136 V/suonsənb/əxeı/6 177 1. This is the data sheet for Molar mass of vapor; 1. Unknown Liquid #:N/A 2. Mass of flask+ cap + rubber band 56.45 g se 3. Temperature of boiling water 97 °C ३ 4. Barometric Pressure 755.7 mmHg 5. Mass of flask + rubber band + cap + condensed vapor_57.88 6. Mass of condensed vapor 7. Volume of flask 243.8 mL 8. Molar Mass of Vapor g/mol What is the molar mass of vapor? O 179200 O 1.79 0179.2 N beatsqudio
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 106QRT
Related questions
Question
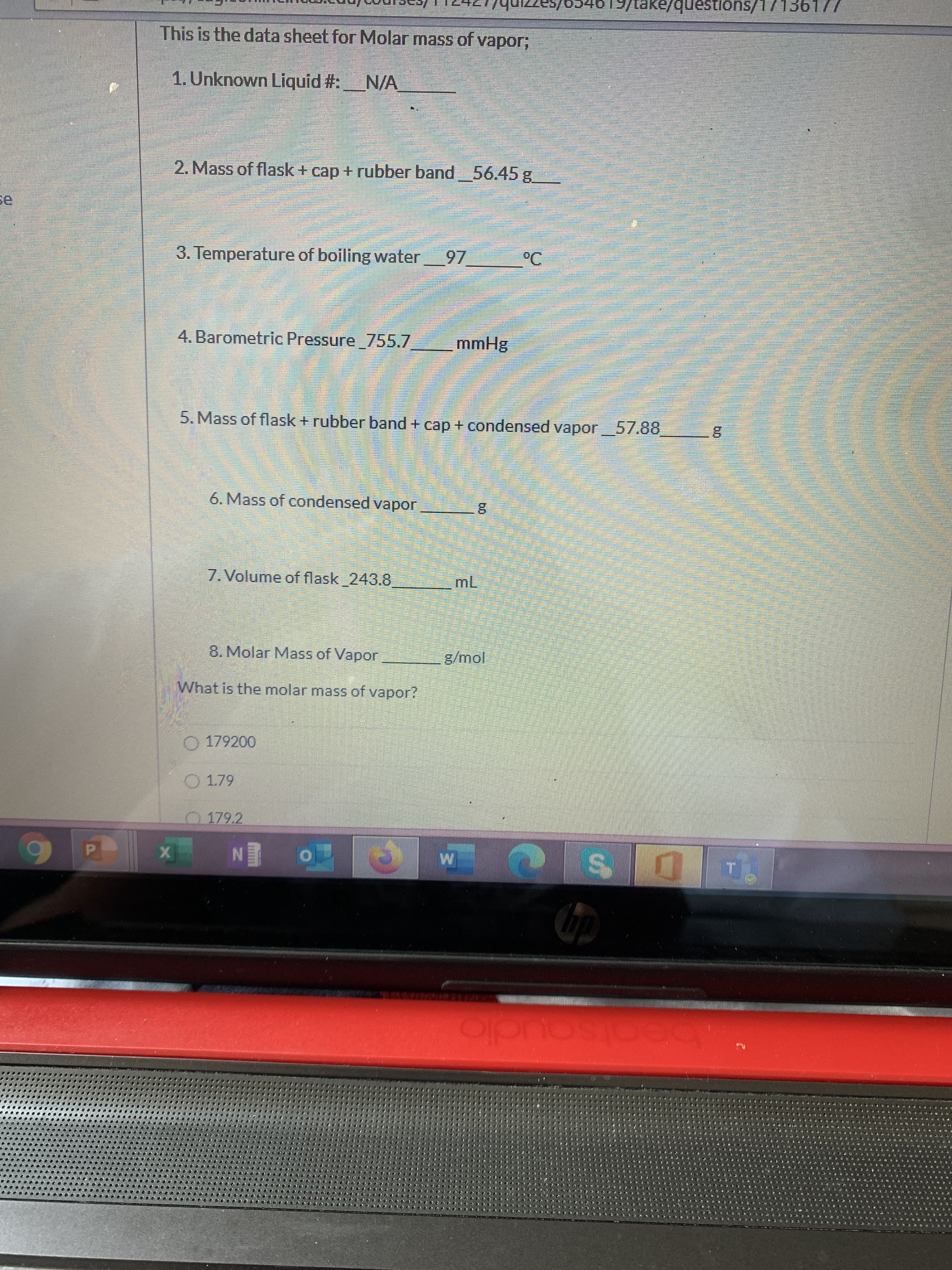
Transcribed Image Text:ons/17136
V/suonsənb/əxeı/6
177
1.
This is the data sheet for Molar mass of vapor;
1. Unknown Liquid #:N/A
2. Mass of flask+ cap + rubber band
56.45 g
se
3. Temperature of boiling water 97
°C
३
4. Barometric Pressure 755.7
mmHg
5. Mass of flask + rubber band + cap + condensed vapor_57.88
6. Mass of condensed vapor
7. Volume of flask 243.8
mL
8. Molar Mass of Vapor
g/mol
What is the molar mass of vapor?
O 179200
O 1.79
0179.2
N
beatsqudio
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 8 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax