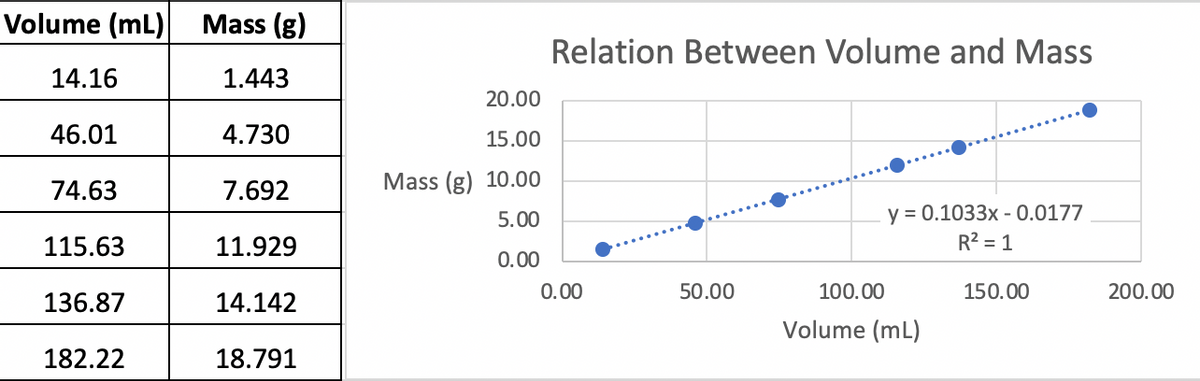
.What is the slope of the line plotted using your data? What are the appropriate units of the slope(remember: the slope is rise over run–what units come from that?)?
ANSWER THE QUESTION BY LOOKING AT THE DATA GIVEN IN THE PHOTO
1.What is the slope of the line plotted using your data? What are the appropriate units of the slope(remember: the slope is rise over run–what units come from that?)?
2.What physical property does the slope represent?
3.What is the y-intercept of the line plotted using your data? What are the units of the y-intercept?
4.Examine the data and the graph carefully and then comment on the accuracy of the y-intercept. What should the y-intercept be? (note: it is possible to force Excel to make a trendline with this feature. This could be useful in the future.)
5.What is the R2value for the graph of the data in Table 1? What does R2measure? Use the value of R2to comment on the precision of the data. Explain your reasoning!
6.Use the equation of the trend line to calculate the expected volume of a sample of the unknown substance with a mass of 47.19g. Show your work using correct units and significant figures for all numbers.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps








