0 ||| a laccd sign in - Search tab . esc Explanation If O CHEMICAL REACTIONS Theoretical yield of chemical reactions 1 https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym96sW-8PcvpF38ZsC... Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H₂O). What is the theoretical yield of sodium chloride formed from the reaction of 2.2 g of hydrochloric acid and 2.0 g of sodium hydroxide? Be sure your answer has the correct number of significant digits in it. Type here to search 1 ? C Check a 2 A ALEKS W # 3 E $ O x10 X 4 O R % BI 40 5 X ? T ^ McGraw-Hill Education Campus X A ALEKS-Shushanik Babayan - Le X + 4- 6 & + 7 144 €3 8 D-II ( Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility n (?) 5:50 PM /19/2022 9 0 DPI :) O O A U 1/5 { 94°F insert Q [ prt sc Shushanik } 1 F ola backspace
0 ||| a laccd sign in - Search tab . esc Explanation If O CHEMICAL REACTIONS Theoretical yield of chemical reactions 1 https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym96sW-8PcvpF38ZsC... Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H₂O). What is the theoretical yield of sodium chloride formed from the reaction of 2.2 g of hydrochloric acid and 2.0 g of sodium hydroxide? Be sure your answer has the correct number of significant digits in it. Type here to search 1 ? C Check a 2 A ALEKS W # 3 E $ O x10 X 4 O R % BI 40 5 X ? T ^ McGraw-Hill Education Campus X A ALEKS-Shushanik Babayan - Le X + 4- 6 & + 7 144 €3 8 D-II ( Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility n (?) 5:50 PM /19/2022 9 0 DPI :) O O A U 1/5 { 94°F insert Q [ prt sc Shushanik } 1 F ola backspace
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.159QP: The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by...
Related questions
Question
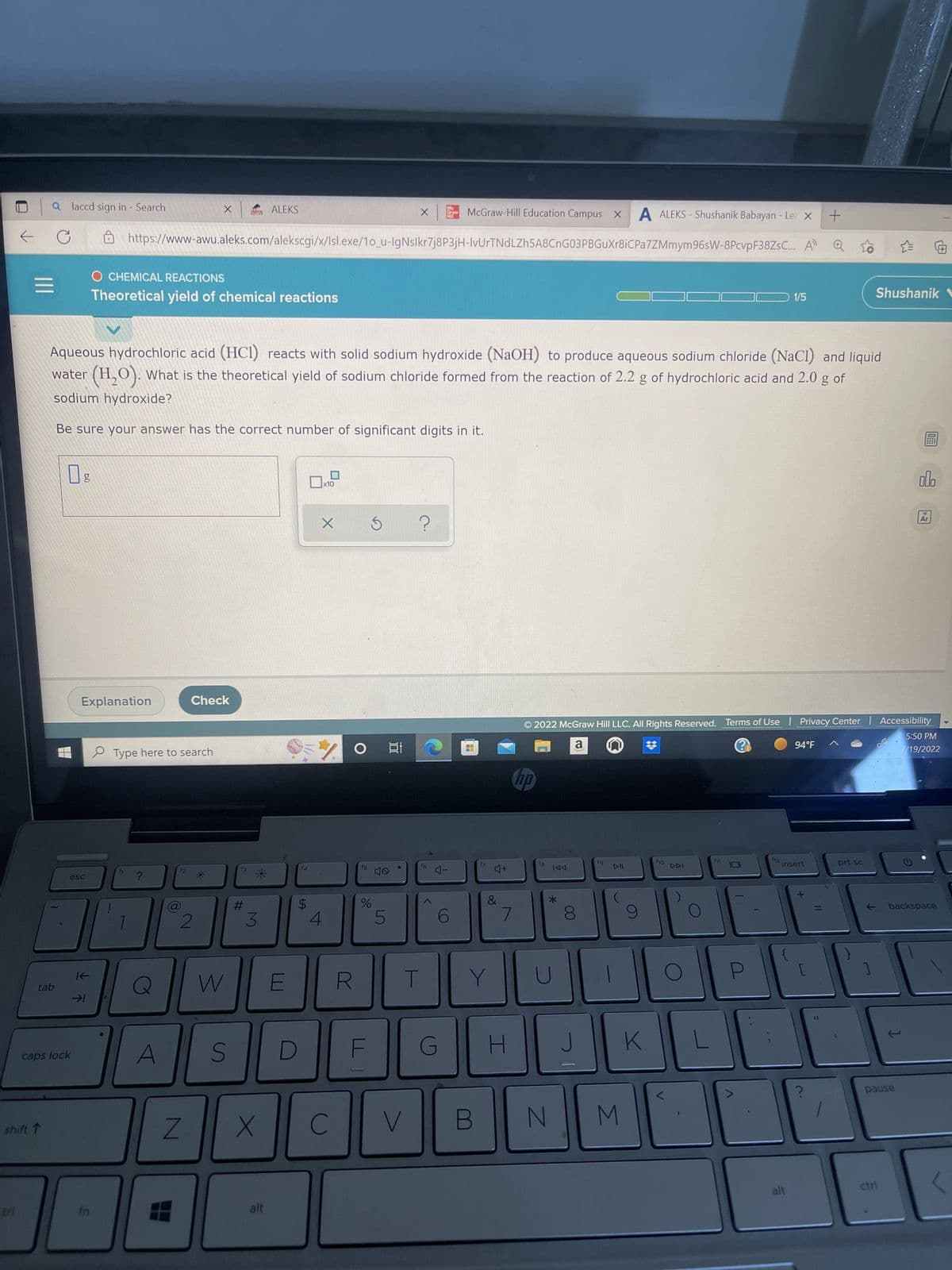
Please answer correct significant fig
Transcribed Image Text:a laccd sign in - Search
C
|||
tab
shift 1
caps lock
esc
Explanation
Kf
→>1
in
O CHEMICAL REACTIONS
Theoretical yield of chemical reactions
Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid
water (H₂O). What is the theoretical yield of sodium chloride formed from the reaction of 2.2 g of hydrochloric acid and 2.0 g of
sodium hydroxide?
Be sure your answer has the correct number of significant digits in it.
Type here to search
?
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym
A
X
12
Check
2
Z
1
W
S
#
*
ALEKS
3
X
alt
E
f4
$
D
4
x10
X
C
O
f5
S
%
F
5
X
V
?
T
Mc
Graw
Hl
f6
4-
McGraw-Hill Education Campus X A ALEKS-Shushanik Babayan - Le X +
96sW-8PcvpF38ZsC... A
6
G
17
&
Y
+
7
I
np
18
৷বব
*
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
M a
5:50 PM
7/19/2022
8
J
fg
DII
N
B
9
K
M
f10
DDI
1/5
f12
alt
94°F
insert
Lad
[
11
(*
prt sc
Shushanik V
L
J
Ⓒ
G
pause
000
18
Ar
backspace
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning