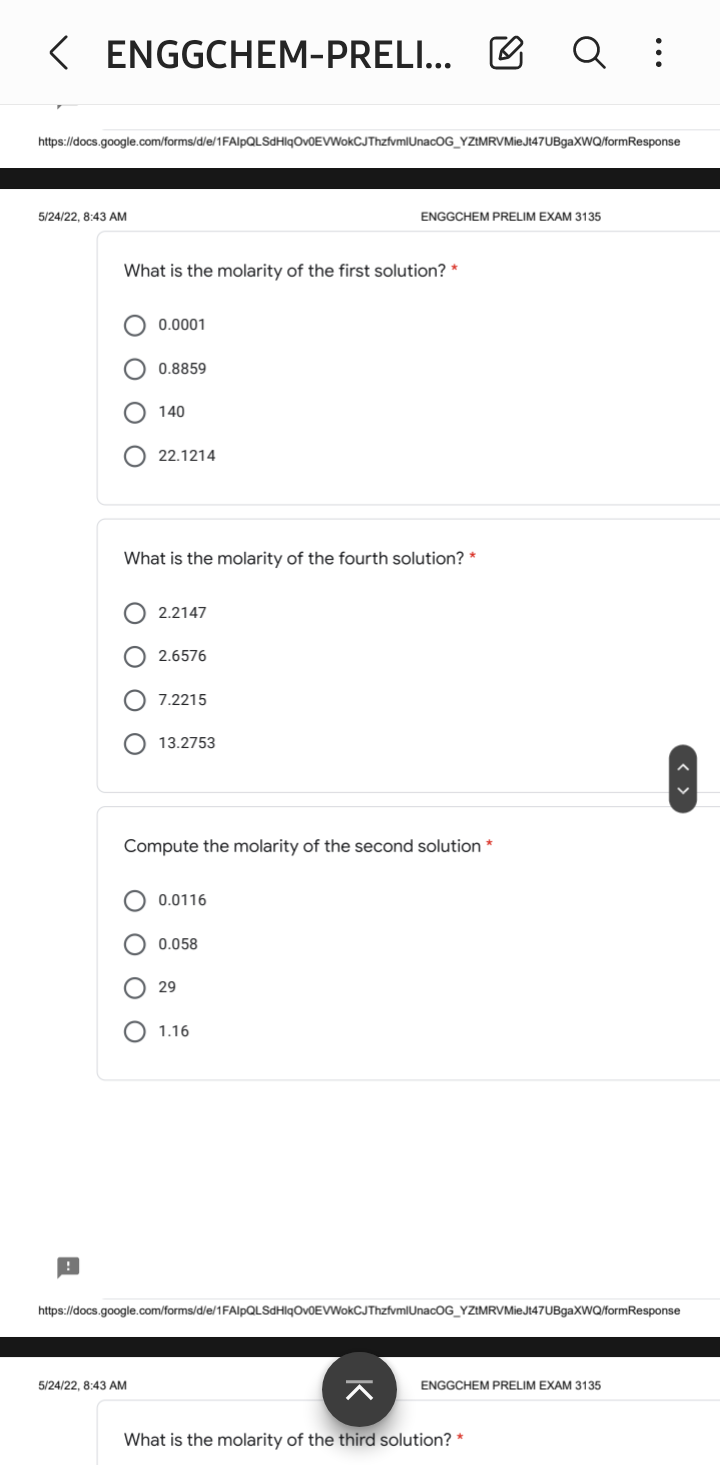
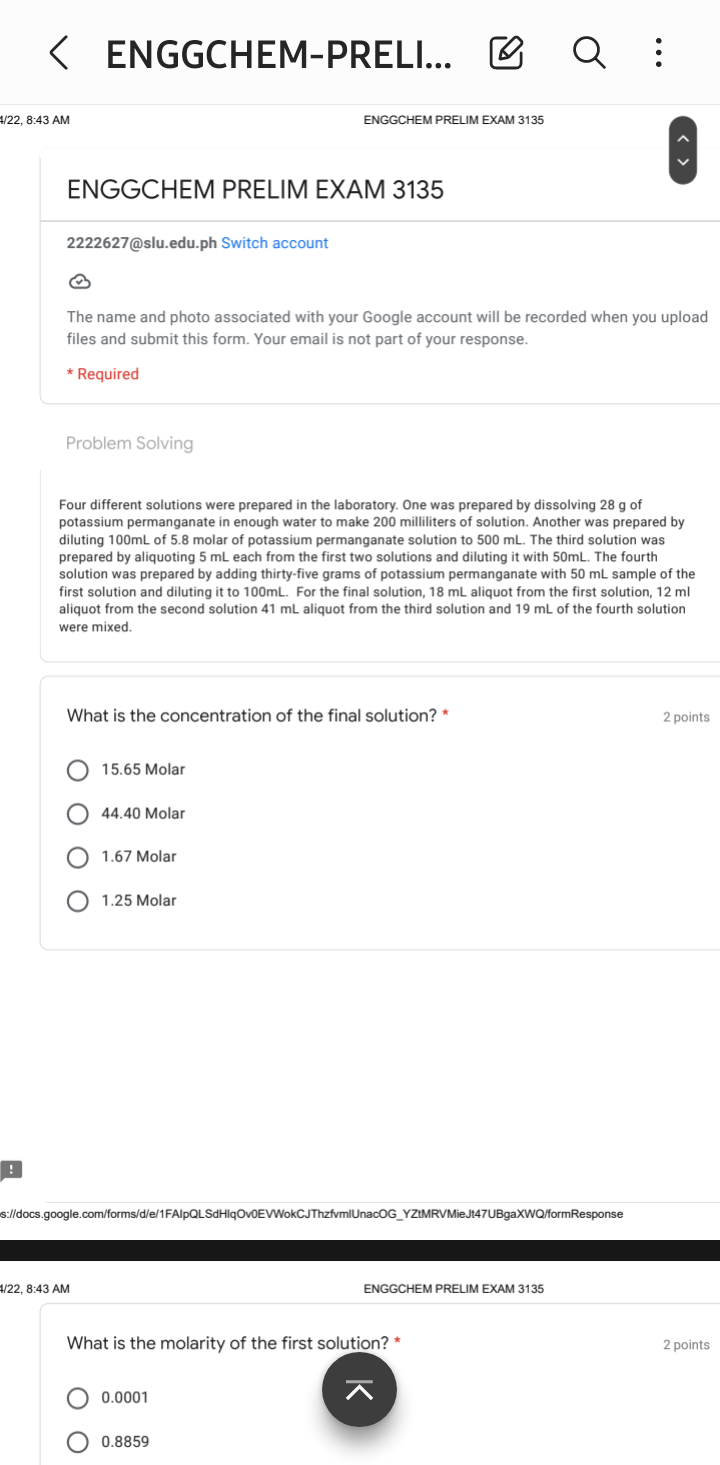
Four different solutions were prepared in the laboratory. One was prepared by dissolving 28 g of potassium permanganate in enough water to make 200 milliliters of solution. Another was prepared by diluting 100ml of 5.8 molar of potassium permanganate solution to 500 mL. The third solution was prepared by aliquoting 5 mL each from the first two solutions and diluting it with 50mL. The fourth solution was prepared by adding thirty-five grams of potassium permanganate with 50 mL sample of the first solution and diluting it to 100mL. For the final solution, 18 mL aliquot from the first solution, 12 ml aliquot from the second solution 41 mL aliquot from the third solution and 19 mL of the fourth solution were mixed.
What is the molarity of the fourt solution?
What is the concentration of the final solution?
Step by step
Solved in 6 steps









