03 Solubility curves can be used to determine if a solution is saturated, unsaturated, or supersaturated. Solubility Curve for KNO, A(n) 150 140 130 solution has reached the limit of the amount of solute that can be dissolved at a given temperature. A(n) 120 $10 100 saturated 90 ed solution is still below the limit of the amount of solute that can be dissolved at a given temperature, and can still have more solute dissolved in it. 70 60 50 unsaturated 40 A(n) 90 20 10 solution has gone above the limit of the amount of solute that can be dissolved at a given temperature. These are very unstable, and often lead to crystallization. 0 0 10 TO 80 90 100 20 30 40 50 60 Temperature (°C) ob obia 211 11. Identify if the solution is saturated, unsaturated, or supersaturated using the solubility curves on the first page. a. 110 grams of potassium nitrate (KNO3) in 100 grams of water at 60°C b. 90 grams of sodium nitrate (NaNO3) in 100 grams of water at 40°C c. 80 grams of ammonium chloride (NH4CI) in 100 grams of water at 70°C Sabbility (grams of sche/100g H0) saturated/ supersaturated
03 Solubility curves can be used to determine if a solution is saturated, unsaturated, or supersaturated. Solubility Curve for KNO, A(n) 150 140 130 solution has reached the limit of the amount of solute that can be dissolved at a given temperature. A(n) 120 $10 100 saturated 90 ed solution is still below the limit of the amount of solute that can be dissolved at a given temperature, and can still have more solute dissolved in it. 70 60 50 unsaturated 40 A(n) 90 20 10 solution has gone above the limit of the amount of solute that can be dissolved at a given temperature. These are very unstable, and often lead to crystallization. 0 0 10 TO 80 90 100 20 30 40 50 60 Temperature (°C) ob obia 211 11. Identify if the solution is saturated, unsaturated, or supersaturated using the solubility curves on the first page. a. 110 grams of potassium nitrate (KNO3) in 100 grams of water at 60°C b. 90 grams of sodium nitrate (NaNO3) in 100 grams of water at 40°C c. 80 grams of ammonium chloride (NH4CI) in 100 grams of water at 70°C Sabbility (grams of sche/100g H0) saturated/ supersaturated
Chapter80: Crystallization: Purification Of Solids
Section: Chapter Questions
Problem 1P
Related questions
Question
can one help with this page? please
Transcribed Image Text:on
m
no3
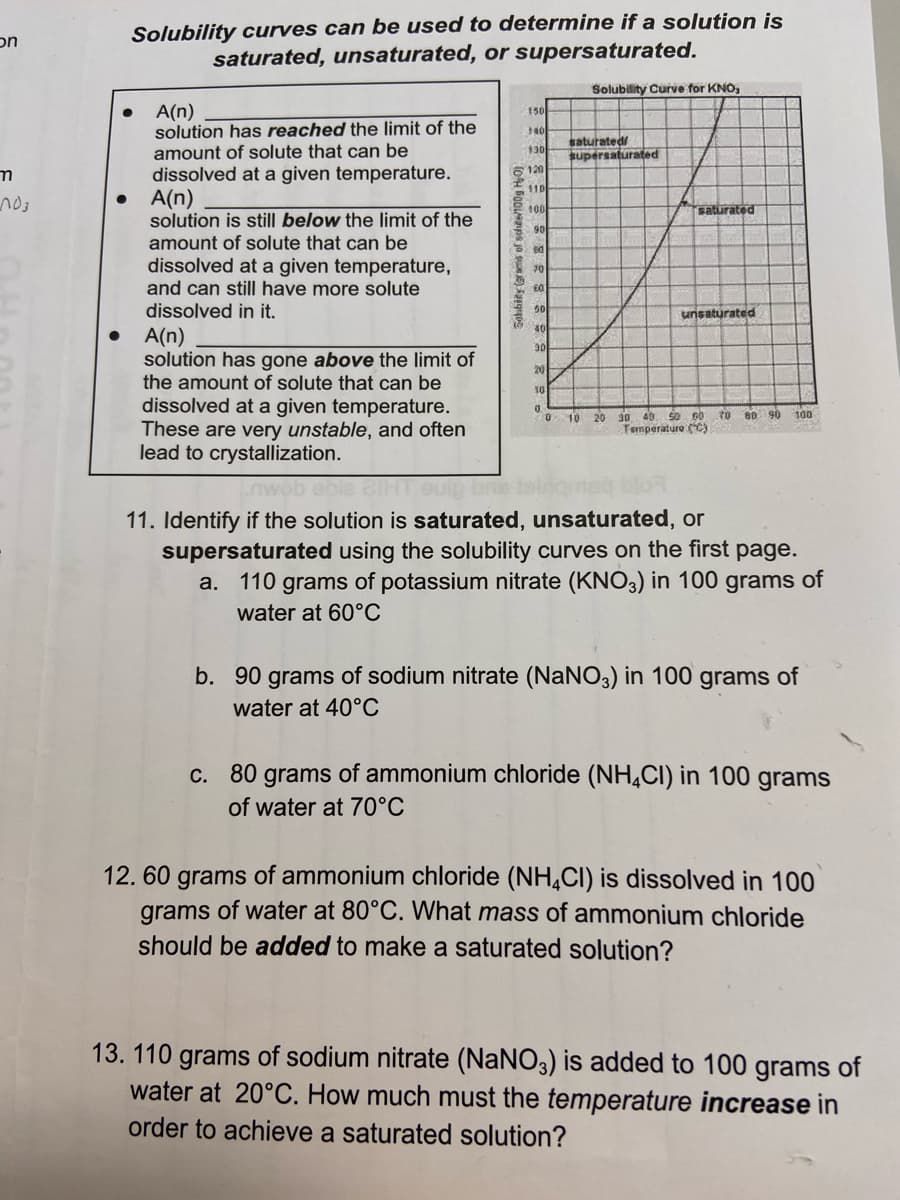
Solubility curves can be used to determine if a solution is
saturated, unsaturated, or supersaturated.
Solubility Curve for KNO,
A(n)
150
140
solution has reached the limit of the
amount of solute that can be
130
dissolved at a given temperature.
6120
$10
A(n)
100
saturated
90
solution is still below the limit of the
amount of solute that can be
dissolved at a given temperature,
and can still have more solute
dissolved in it.
ed
70
unsaturated
A(n)
30
21
10
solution has gone above the limit of
the amount of solute that can be
dissolved at a given temperature.
These are very unstable, and often
lead to crystallization.
0
100
0 10 20 30 40 50 60 70 80 90
Temperature (°C)
BETA
vob obie 21HT uip
11. Identify if the solution is saturated, unsaturated, or
supersaturated using the solubility curves on the first page.
a. 110 grams of potassium nitrate (KNO3) in 100 grams of
water at 60°C
b. 90 grams of sodium nitrate (NaNO3) in 100 grams of
water at 40°C
c. 80 grams of ammonium chloride (NH4CI) in 100 grams
of water at 70°C
12.60 grams of ammonium chloride (NH4CI) is dissolved in 100
grams of water at 80°C. What mass of ammonium chloride
should be added to make a saturated solution?
13. 110 grams of sodium nitrate (NaNO3) is added to 100 grams of
water at 20°C. How much must the temperature increase in
order to achieve a saturated solution?
●
●
Sohbility (grams c
saturated/
supersaturated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning