1) Based on the amounts of copper metal and nitric acid you used in the first reaction, calculate the number of moles of HNO3 that are in excess. Concentrated nitric acid has a concentration of 15.8 M. noitanen garwollol odi yd bios bin (M 01) sulthy O.HP OMSOPOR blov wo blow noites tent TON apoy melqxdl Slom de to upora 2) Using the moles of HNO3 you calculated and the moles of Cu(NO3)2 produced from the first reaction, calculate the total mass of sodium carbonate needed for the second and third reactions. Did you add enough sodium carbonate in the experiment?
1) Based on the amounts of copper metal and nitric acid you used in the first reaction, calculate the number of moles of HNO3 that are in excess. Concentrated nitric acid has a concentration of 15.8 M. noitanen garwollol odi yd bios bin (M 01) sulthy O.HP OMSOPOR blov wo blow noites tent TON apoy melqxdl Slom de to upora 2) Using the moles of HNO3 you calculated and the moles of Cu(NO3)2 produced from the first reaction, calculate the total mass of sodium carbonate needed for the second and third reactions. Did you add enough sodium carbonate in the experiment?
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.20QAP
Related questions
Question
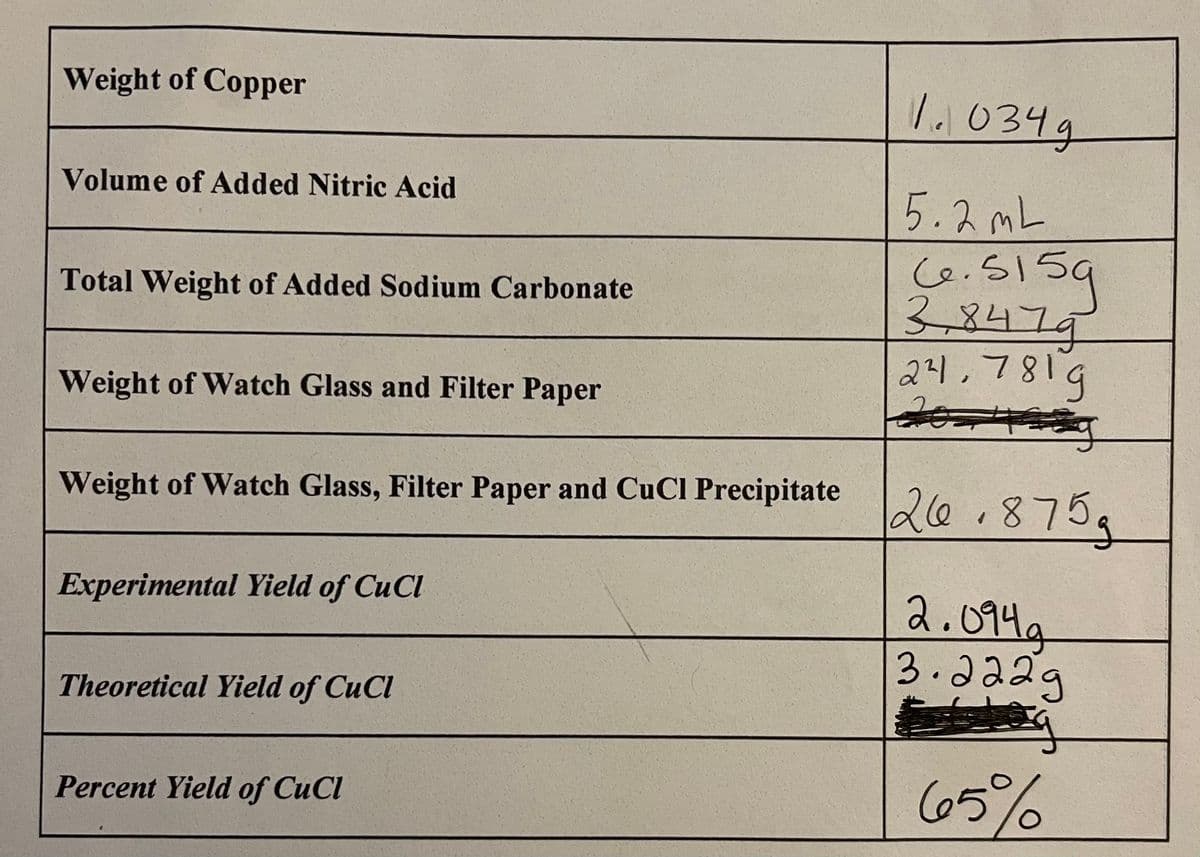
Transcribed Image Text:Weight of Copper
Volume of Added Nitric Acid
Total Weight of Added Sodium Carbonate
Weight of Watch Glass and Filter Paper
Weight of Watch Glass, Filter Paper and CuCl Precipitate 26.875g
Experimental Yield of CuCl
Theoretical Yield of CuCl
1.034g
5.2mL
6.5159
3,8479
24,7819
국
Percent Yield of CuCl
2.094a
3.2229
€
65%
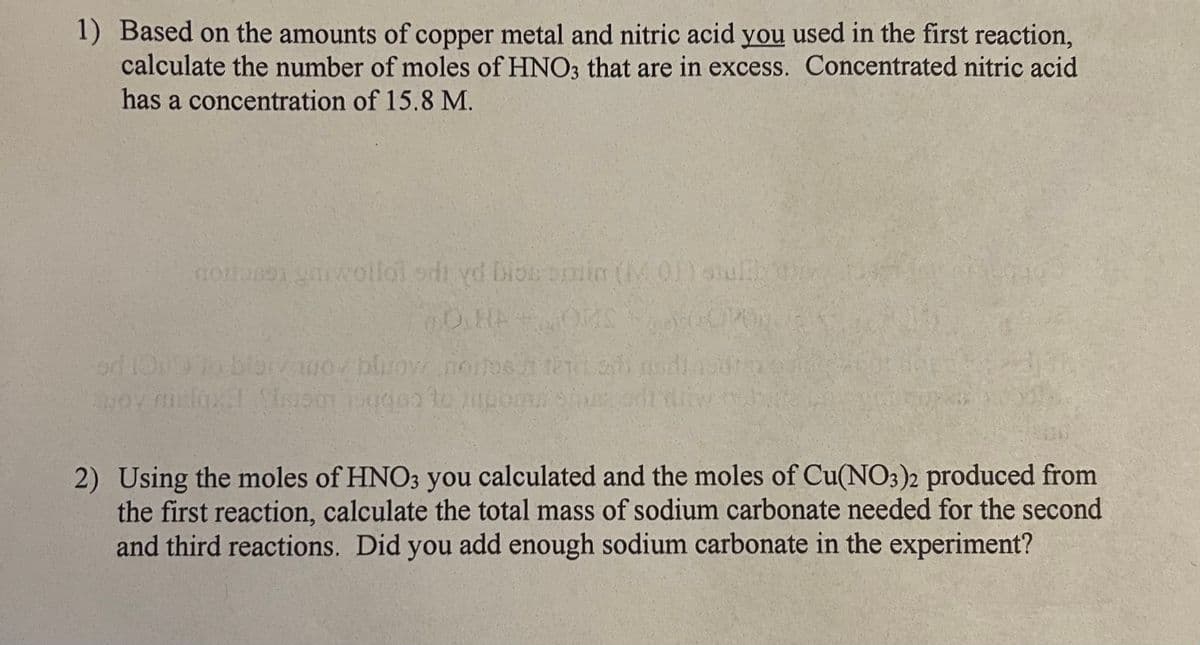
Transcribed Image Text:1) Based on the amounts of copper metal and nitric acid you used in the first reaction,
calculate the number of moles of HNO3 that are in excess. Concentrated nitric acid
has a concentration of 15.8 M.
noitasen ymwollol odi yd bios bimin (01) shulth dy
OHA
OMS
od 1019 to blair wor blow noitesh te shi di a
moy nielqx! Som do to zupom sin
2) Using the moles of HNO3 you calculated and the moles of Cu(NO3)2 produced from
the first reaction, calculate the total mass of sodium carbonate needed for the second
and third reactions. Did you add enough sodium carbonate in the experiment?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
