1) Define the following terms: titration, equivalence point, end point, titration curve. 10 Vekm el b aded (l) 20 30 2) Based on Figure 1, where does the pH begin and where does the pH end? 3) Based on figure one, what is the independent variable? What is the de pendent variable?
1) Define the following terms: titration, equivalence point, end point, titration curve. 10 Vekm el b aded (l) 20 30 2) Based on Figure 1, where does the pH begin and where does the pH end? 3) Based on figure one, what is the independent variable? What is the de pendent variable?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter16: Acid-base Equilibria
Section: Chapter Questions
Problem 16.149QP: A solution of weak base is titrated to the equivalence point with a strong acid. Which one of the...
Related questions
Question
Helpppp
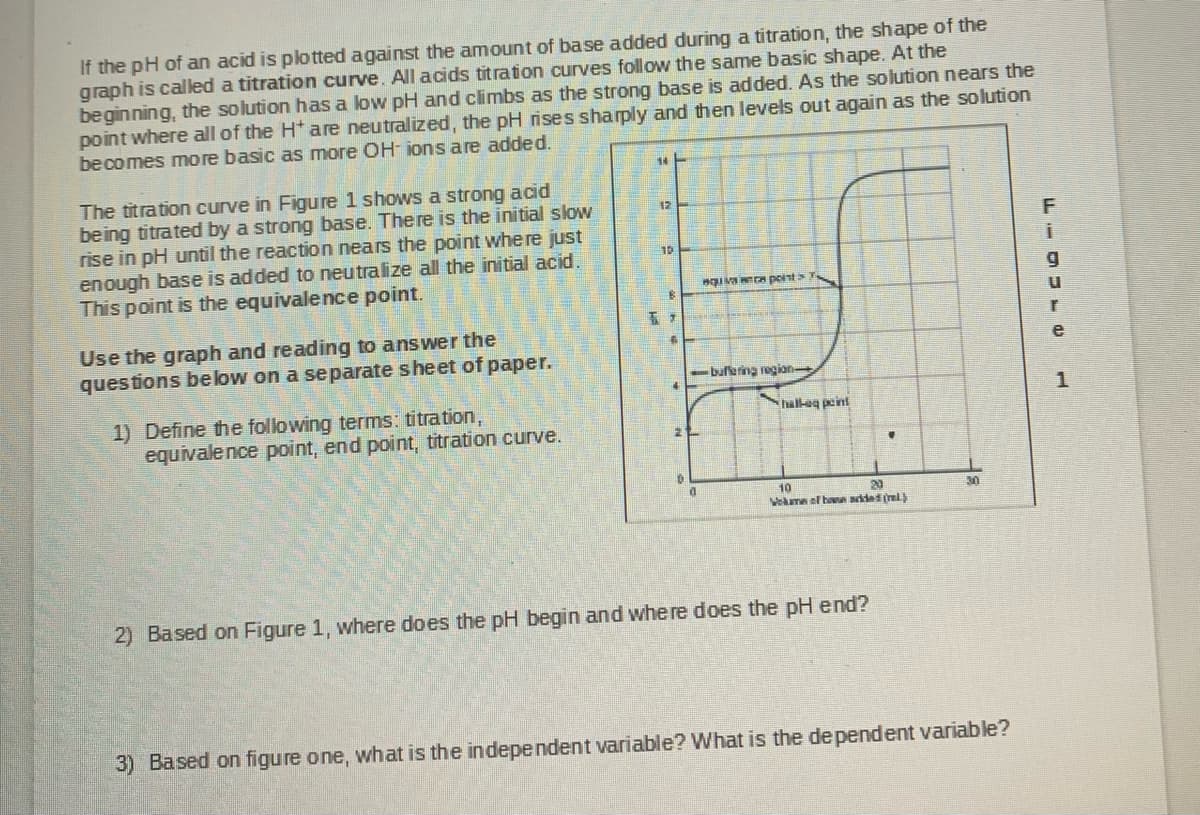
Transcribed Image Text:If the pH of an acid is plotted against the amount of base added during a titration, the shape of the
graph is caled a titration curve. All acids titration curves follow the same basic shape. At the
beginning, the solution has a low pH and climbs as the strong base is added. As the solution nears the
point where all of the H* are neutralized, the pH rises sharply and then levels out again as the solution
becomes more basic as more OH- ions are added.
The titration curve in Figure 1 shows a strong acid
being titrated by a strong base. There is the initial slow
rise in pH until the reaction nears the point where just
enough base is added to neutralize all the initial acid.
This point is the equivalence point.
12
10
IvA point
Use the graph and reading to answer the
ques tions below on a separate sheet of paper.
-buffering region
1) Define the following terms: titration,
equivalence point, end point, titration curve.
hall-eg peint
10
Vekam el han adad (ml
20
30
2) Based on Figure 1, where does the pH begin and where does the pH end?
3) Based on figure one, what is the independent variable? What is the de pendent variable?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole