O Itis a titration curve with a sigmoidal shape. O The red line is located at the equivalence point. O The red line is located where the concentration of the dissociated hydrogen ions is equal to the undissociated acid concentration. O The red line is located where the moles of hydroxide ions and the dissociated hydrogen ions are equal.
O Itis a titration curve with a sigmoidal shape. O The red line is located at the equivalence point. O The red line is located where the concentration of the dissociated hydrogen ions is equal to the undissociated acid concentration. O The red line is located where the moles of hydroxide ions and the dissociated hydrogen ions are equal.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 105AP: . Write the formulas for three combinations of weak acid and salt that would act as buffered...
Related questions
Question
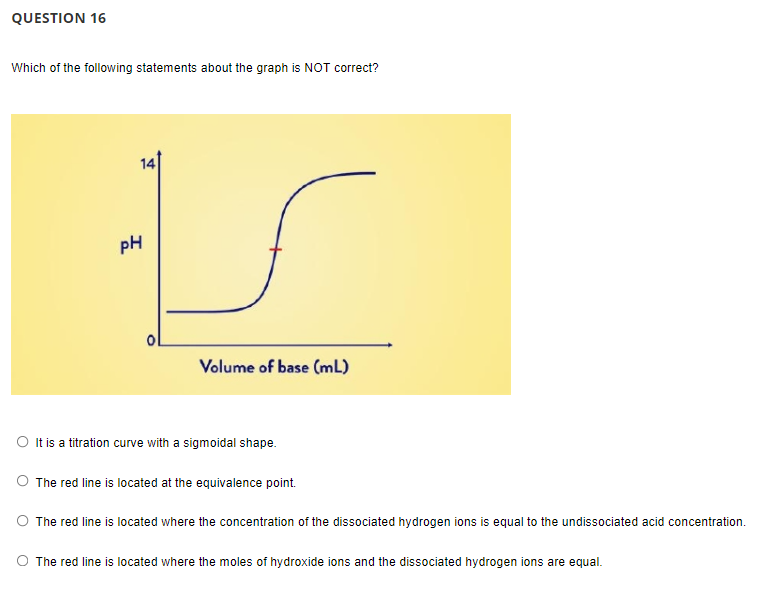
Transcribed Image Text:QUESTION 16
Which of the following statements about the graph is NOT correct?
14
pH
Volume of base (mL)
O It is a titration curve with a sigmoidal shape.
The red line is located at the equivalence point.
O The red line is located where the concentration of the dissociated hydrogen ions is equal to the undissociated acid concentration.
The red line is located where the moles of hydroxide ions and the dissociated hydrogen ions are equal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning