1- During a chemical reaction, which of the following statements is ALWAYS true? I) A system loses energy to its surroundings. II) A system gains energy from its surroundings. iii) The kinetic energy of the system equals the potential energy of the surroundings. IV)The total energy of a system and its surroundings remains constant.
1- During a
I) A system loses energy to its surroundings.
II) A system gains energy from its surroundings.
iii) The kinetic energy of the system equals the potential energy of the surroundings.
IV)The total energy of a system and its surroundings remains constant.
2- Which of the following properties is NOT required to determine the amount of thermal energy needed to raise the temperature of a substance?
1- mass
2- volume
3-temperature change
4-specific heat capacity
3-Which of the following is true of endothermic reactions?
A) They have a positive change in enthalpy.
B)They release energy.
C)They have energy terms written on the product side of the reaction.
D)They can be identified by an increase in temperature of the surroundings.
4- How much energy is absorbed by a 2.5 g substance that has a specific heat capacity of 0.58 J/gC and undergoes a temperature increase of 1.2C?
A)1.2 J
B)1.7 J
C)5.2 J
D)0.8 J
5- Given the thermochemical equation below( will be labelled as Q5), what is the enthalpy change when 10.1 g of hydrogen gas is reacted with excess oxygen?
1- 2860 kJ
2) -2860 kJ
3) 1430 kJ
4) -1430 kJ
6- Given the formation reaction represented below(will be labelled as q6), which of the following substances in the reaction has a molar enthalpy of formation equal to 0?
a) C3H8 (propane)
b) O2 (oxygen gas)
c) H2O (water)
d) CO2 (carbon dioxide)
E) c) and d)
F)None of the above
7- Which of the following statements are FALSE?
1- The molar enthalpy of formation of mercury gas, Hg(g), at SATP, is equal to 0.
2- When using molar enthalpies of formation to calculate the molar enthalpy change of a reaction, it is important that the equation you are analyzing is accurately balanced.
C- Breaking bonds requires energy, forming bonds releases energy.
4- Calorimetry cannot be used for all reactions.
8-Explain why the option you chose in question 7 is false. The answer should be at least 2 sentences long.
9- What is the standard enthalpy of formation for aqueous hydrogen peroxide (H2O2)? Answer with a number and its units only.
10- What is the standard enthalpy of formation for aqueous hydrogen peroxide (H2O2)? Answer with a number and its units only.
11- A particular reaction has a molar enthalpy value of 1713.8 Joules. If 2 moles of reactant is used in a calorimeter filled with 100 mL of water, the water reaches a final temperature of 30 degrees celsius. What was the initial temperature of the water? Round your answer to 1 decimal place, if necessary. Answer with the number only, no units
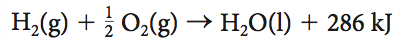
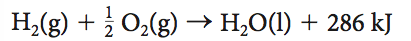
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images









