1- While cleaning out a laboratory shelf, you have made a mistake in labeling two bottles of ethanol and acetic acid. To identify the molecular structure of the contents of the bottle, samples were submitted for analysis using infrared spectroscopy, based on the IR results, draw the molecular structure of the molecule that would correspond to the spectrum for that compound. Justify your answer
1- While cleaning out a laboratory shelf, you have made a mistake in labeling two bottles of ethanol and acetic acid. To identify the molecular structure of the contents of the bottle, samples were submitted for analysis using infrared spectroscopy, based on the IR results, draw the molecular structure of the molecule that would correspond to the spectrum for that compound. Justify your answer
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 6P
Related questions
Question
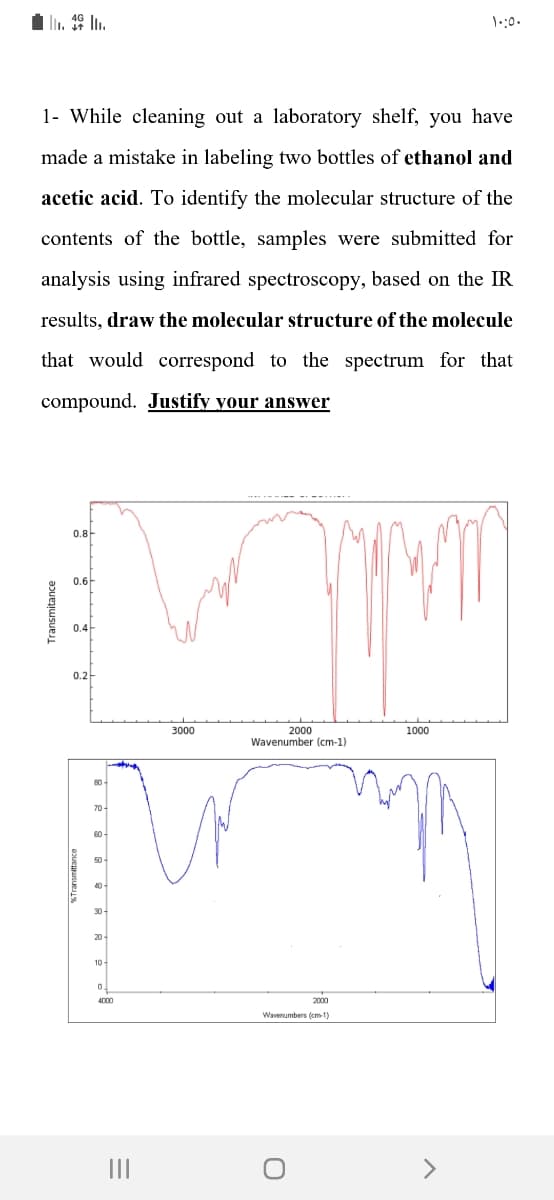
Transcribed Image Text:1- While cleaning out a laboratory shelf, you have
made a mistake in labeling two bottles of ethanol and
acetic acid. To identify the molecular structure of the
contents of the bottle, samples were submitted for
analysis using infrared spectroscopy, based on the IR
results, draw the molecular structure of the molecule
that would correspond to the spectrum for that
compound. Justify your answer
0.8
0.6-
0.4-
0.2
3000
2000
Wavenumber (cm-1)
1000
80
70
60
40
30-
20
10
2000
Wavenumbers (cm-1)
II
Transmitance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning